Professional Documents
Culture Documents
Intro To Carbocations - Key
Uploaded by
elabachyanOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Intro To Carbocations - Key
Uploaded by
elabachyanCopyright:
Available Formats
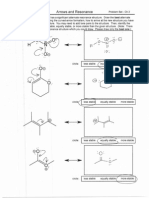
Carbocation Stability
1. Draw the structure of the methyl cation, CH3+. Describe the hybridization, geometry, and orbitals in this
species.
H
C
T he carbon here is sp2-hybridized, with trigonal planar geometry
( 120-degree bond angles) and a vacant 2p orbital on carbon.
2. Each of the following carbocations is significantly more stable than the methyl cation. Explain why each of
these species is especially stable. You should use both molecular orbital and resonance arguments in your
explanations.
resonance-stabilized
resonance-stabilized
H3C
H2
C CH2
H2C
( no resonance stabilization)
H
C
H
CH2
2pC
H2C
C-H
C
H
CH2
H3C
CH2
H3C
CH2
Hyperconjugation ( overlap of
H
2pC with the neighboring C-H )
H
helps to stabilize the carbocation;
the overlap is not particularly
Overlap of C-C with the
ef f ective but at least it makes it
vacant 2p orbital is more
more stable than the methyl
stabilizing than mere
cation!
hyperconjugation ( C-C is
a higher-energy donor
than C-H ).
H3C
T he lone pair electrons on
oxygen ( nO) are even higher
in energy than pC-C, thus are
even better donors to help
stabilize the carbocation.
3. Rank the above cations from least to most stable. Again, use both MO and resonance arguments to
explain the relative order of stability.
Since the energy of the f illed orbitals that overlap with the vacant 2p orbital on carbon is:
C-H
<
C-C
< nO
Then the stability of the carbocations themselves has the same ranking; in other words:
H3C
H2
C CH2
<
H2C
C
H
CH2
<
H3C
CH2
33
2014-06-05 15:25:00
1/2
Intro to Carbocations KEY.pdf (#2)
Carbocation Formation and Rearrangement
1. Each of the following alkyl halides can form a carbocation. Draw a curved arrow mechanism for the single
step of carbocation formation, and show the carbocation product.
Cl
Cl
Cl
2. Each of the following alkyl halides can form a carbocation. That carbocation can then rearrange to form a
more stable carbocation. Show a curved arrow mechanism for the protonation and rearrangement steps.
Make sure you understand why the rearrangement takes place!
Br
Br
2014-06-05 15:25:00
2/2
Intro to Carbocations KEY.pdf (2/2)
You might also like
- Hyperconjugation - Dr. Akshay ShuklaDocument26 pagesHyperconjugation - Dr. Akshay ShuklawaqasNo ratings yet
- CHM 1321 Assignment #2 - : AnswersDocument11 pagesCHM 1321 Assignment #2 - : AnswersSara YuenNo ratings yet
- Chapter 11Document17 pagesChapter 11abubakarabubakarbah563No ratings yet
- Ch221 Class 8 Chapter 4: Stereochemistry of Alkanes and Cycloalkanes, ContinuedDocument11 pagesCh221 Class 8 Chapter 4: Stereochemistry of Alkanes and Cycloalkanes, ContinuedSanjay Mani TripathiNo ratings yet
- Alkanes: HH H H H H H H Staggered EclipsedDocument5 pagesAlkanes: HH H H H H H H Staggered EclipsedCamille AdleNo ratings yet
- Organic Effect - 15.3.2020Document26 pagesOrganic Effect - 15.3.2020Roban SinghNo ratings yet
- Hydrocarbons Final PDFDocument74 pagesHydrocarbons Final PDFAnurag SahuNo ratings yet
- Chapter 2. Alkanes and Cycloalkanes: Introduction To Hydrocarbons 2.1: Classes of HydrocarbonsDocument28 pagesChapter 2. Alkanes and Cycloalkanes: Introduction To Hydrocarbons 2.1: Classes of HydrocarbonsRizki IndahNo ratings yet
- Problems For Chapter 1 & 2 ANSWERS: 2xH 2 2xN 10 O 6Document6 pagesProblems For Chapter 1 & 2 ANSWERS: 2xH 2 2xN 10 O 6JibrilAttawarahNo ratings yet
- Practice Questions-Conformational AnalysisDocument4 pagesPractice Questions-Conformational AnalysisHarry Zgambo100% (1)
- Carbocations 2Document16 pagesCarbocations 2Surabhi BansalNo ratings yet
- Chapter 2Document28 pagesChapter 2Adnan ZahirovicNo ratings yet
- Unit I PCPDocument60 pagesUnit I PCPsuranjana26No ratings yet
- CH1 C02-Structural and Molecular Organic Chemistry 2014Document22 pagesCH1 C02-Structural and Molecular Organic Chemistry 2014xapodi8776No ratings yet
- 226 Alkene Rxns LecDocument12 pages226 Alkene Rxns LecKaaya GodfreyNo ratings yet
- Alkenes - 4Document49 pagesAlkenes - 4Bag CookNo ratings yet
- Alkenes OverviewDocument62 pagesAlkenes OverviewMd KhanNo ratings yet
- Final Exam KeyDocument12 pagesFinal Exam KeykitthiNo ratings yet
- Alkenes - 4Document49 pagesAlkenes - 4Bag CookNo ratings yet
- Physical Organic Chemistry Chapter ThreeDocument40 pagesPhysical Organic Chemistry Chapter ThreeMULUKEN TILAHUNNo ratings yet
- Cuaderno de Trabajo - 2019-2Document35 pagesCuaderno de Trabajo - 2019-2Monica BravoNo ratings yet
- CHM 1321 Assignment 4 AnswersDocument12 pagesCHM 1321 Assignment 4 AnswersSara YuenNo ratings yet
- Long Exam 1Document8 pagesLong Exam 1Allan DNo ratings yet
- Bansal Classes Organic Chemistry Study Material For IIT JEEDocument477 pagesBansal Classes Organic Chemistry Study Material For IIT JEEAditya Kavuluri40% (5)
- Reaction MechanismDocument68 pagesReaction MechanismSiddarth Singh73% (11)
- CL CL: Hex-1-En-4-Yne or 1-Hexen-4-YneDocument4 pagesCL CL: Hex-1-En-4-Yne or 1-Hexen-4-YneSamuel Espinoza GarciaNo ratings yet
- Cuaderno de Trabajo - 2019-2Document35 pagesCuaderno de Trabajo - 2019-2Monica BravoNo ratings yet
- Hydrocarbons 1Document26 pagesHydrocarbons 1Gowri ShankarNo ratings yet
- Molecular RearrangementsDocument158 pagesMolecular RearrangementsMax TNo ratings yet
- Ch1 2 3 ExercisesDocument11 pagesCh1 2 3 ExercisesMancini100% (1)
- Organic Chemistry 2021Document26 pagesOrganic Chemistry 2021xapodi8776No ratings yet
- Chapter 3Document27 pagesChapter 3Siddarth PalletiNo ratings yet
- Carbanions and Enolizations-MetaDocument17 pagesCarbanions and Enolizations-Metafahimahmed3275No ratings yet
- Reasoning Questions in Organic ChemistryDocument10 pagesReasoning Questions in Organic ChemistryAasthaNo ratings yet
- CHM 2210 Practice Ex I If 12Document10 pagesCHM 2210 Practice Ex I If 12Shaima MossamatNo ratings yet
- E1 and E2 Reactions 2Document23 pagesE1 and E2 Reactions 2Vidhu PandeyNo ratings yet
- 1.5 Introduction To Organic ChemistryDocument14 pages1.5 Introduction To Organic Chemistrymaya 1DNo ratings yet
- Chapter 11Document26 pagesChapter 11Eshita SharmaNo ratings yet
- Conjugation and HyperconjugationDocument3 pagesConjugation and Hyperconjugationmschlecht100% (1)
- Graviity Academy Organic Chemistry: Cleavage of Covalent BondDocument22 pagesGraviity Academy Organic Chemistry: Cleavage of Covalent BondAmber BorseNo ratings yet
- The Pinacol-Pinacolone RearrangementDocument9 pagesThe Pinacol-Pinacolone RearrangementParag MehtaNo ratings yet
- Chemistry 10006Document27 pagesChemistry 10006Shai Shazza GrossNo ratings yet
- DR R D Shah 2Document43 pagesDR R D Shah 2yur fanNo ratings yet
- Alkanes Lecture - 1Document54 pagesAlkanes Lecture - 1hagshhsiauhagah516525No ratings yet
- Chap. 5 Reactive Intermediates: Energy SurfaceDocument20 pagesChap. 5 Reactive Intermediates: Energy SurfaceAnil KumarNo ratings yet
- Chapt 10Document33 pagesChapt 10bhisma.nugerahNo ratings yet
- Organic Chemistry: William H. Brown & Christopher S. FooteDocument67 pagesOrganic Chemistry: William H. Brown & Christopher S. Footealyssa_marie_keNo ratings yet
- CH1O3 Questions PDFDocument52 pagesCH1O3 Questions PDFPrince T MashandaNo ratings yet
- Answer Key: Chemistry 206 First Hour ExaminationDocument9 pagesAnswer Key: Chemistry 206 First Hour Examinationsudipta88No ratings yet
- Combined OrganicDocument82 pagesCombined OrganicSachin KumarNo ratings yet
- IB Topic 10: Organic Chemistry Practice QuestionsDocument36 pagesIB Topic 10: Organic Chemistry Practice Questionshunarsandhu50% (2)
- Ch4,5 Alkene IDocument42 pagesCh4,5 Alkene INizarNo ratings yet
- Chapter 6 NotesDocument21 pagesChapter 6 NotesJesús Adrián Gómez OrtizNo ratings yet
- Solution Manual For Chemistry An Atoms Focused Approach 3rd Edition Thomas R Gilbert Rein V Kirss Stacey Lowery Bretz Natalie FosterDocument37 pagesSolution Manual For Chemistry An Atoms Focused Approach 3rd Edition Thomas R Gilbert Rein V Kirss Stacey Lowery Bretz Natalie FosterChristianGonzalezsrybm100% (82)
- XXIVth International Congress of Pure and Applied Chemistry: Plenary and Main Section Lectures Presented at Hamburg, Federal Republic of Germany, 2–8 September 1973From EverandXXIVth International Congress of Pure and Applied Chemistry: Plenary and Main Section Lectures Presented at Hamburg, Federal Republic of Germany, 2–8 September 1973No ratings yet
- Critical Evaluation of Equilibrium Constants Involving 8-Hydroxyquinoline and Its Metal Chelates: Critical Evaluation of Equilibrium Constants in Solution: Part B: Equilibrium Constants of Liquid-Liquid Distribution SystemsFrom EverandCritical Evaluation of Equilibrium Constants Involving 8-Hydroxyquinoline and Its Metal Chelates: Critical Evaluation of Equilibrium Constants in Solution: Part B: Equilibrium Constants of Liquid-Liquid Distribution SystemsNo ratings yet
- Organometallic Chemistry: Plenary Lectures Presented at the Fourth International Conference on Organometallic ChemistryFrom EverandOrganometallic Chemistry: Plenary Lectures Presented at the Fourth International Conference on Organometallic ChemistryF. G. A. StoneNo ratings yet
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- A New Method of Salinometry-A4Document7 pagesA New Method of Salinometry-A4elabachyanNo ratings yet
- Equilibrium Practice Problems ADocument2 pagesEquilibrium Practice Problems AelabachyanNo ratings yet
- ResearchLog#1 Book LIB1 SheldonDocument3 pagesResearchLog#1 Book LIB1 SheldonelabachyanNo ratings yet
- 0 - Guidelines For Laboratory NotebookDocument1 page0 - Guidelines For Laboratory NotebookelabachyanNo ratings yet
- Lib 1 Assignment: Using Reference Resources For Presearch: InstructionsDocument2 pagesLib 1 Assignment: Using Reference Resources For Presearch: InstructionselabachyanNo ratings yet
- EvaluatingWebInfo Worksheet LIB1Document1 pageEvaluatingWebInfo Worksheet LIB1elabachyanNo ratings yet
- Assignment Database1 NewsMag Sheldon 1Document3 pagesAssignment Database1 NewsMag Sheldon 1elabachyanNo ratings yet
- Assignment 9 2 Alkenes Addition Reactions KeyDocument5 pagesAssignment 9 2 Alkenes Addition Reactions KeyelabachyanNo ratings yet
- Problem Set - CH 2 KEYDocument3 pagesProblem Set - CH 2 KEYelabachyanNo ratings yet