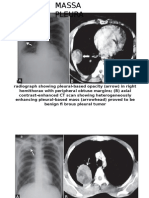
Professional Documents
Culture Documents
Meyer 1982 Brine Shimp Assay
Meyer 1982 Brine Shimp Assay
Uploaded by
swordskylark0 ratings0% found this document useful (0 votes)
13 views4 pagesBrine Shrimp (Artemia Salina) Assay by Meyer, 1982
Original Title
48. Meyer 1982 Brine Shimp Assay
Copyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentBrine Shrimp (Artemia Salina) Assay by Meyer, 1982
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
13 views4 pagesMeyer 1982 Brine Shimp Assay
Meyer 1982 Brine Shimp Assay
Uploaded by
swordskylarkBrine Shrimp (Artemia Salina) Assay by Meyer, 1982
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You are on page 1of 4
1982, Vol. 45, pp. 31-34, © Hippokrates Verlag GmbH
Jouralot Planta
Medicinal
medica
Plant Research
Brine Shrimp: A Convenient General Bioassay for
Active Plant Constituents
B.N. Meyer, N. R. Ferrigni*, J. E. Putnam, L. B. Jacobsen, D. E. Nichols and J. L. McLaughlin
Department af Mecicinal Chemistry and Pharmacognosy, School of Pharmacy and Pharmacal Sciences, and
oll Cuture Laboratory, Purdue Cancer Center, Purdue University, West Lafayette, IN 47907
Received: January 10, 1982
Key Word Index:
Artemia salina; Brine shrimp; Bioassay methods;
Euphorbiaceae seeds; 9KB and 9PS cytotoxicities.
Abstract
A method, utilizing brine shrimp (Artemia salina
Leac), is proposed as a simple bioassay for natural
product research, The procedure determines LCs)
values in ug/ml of active compounds and extracts in
the brine medium. Activities of a broad range of
known active compounds are manifested as toxicity
to the shrimp. Screening results with seed extracts of
41 species of Euphorbiaceae were compared with
9KB and 9PS cytotoxicities. The method is rapid, re-
liable, inexpensive, and convenient as an in-house
general bioassay tool.
Introduction
Many new natural compounds are isolated, cha-
racterized, and published without any biological te~
sting whatsoever. Their useful biological activities
can remain unknown for years. Without accompany-
ing biological data, the discovery of new medicinal
plant constituents is nothing more than pure phyto-
chemistry. Yet, the search for specific pharmacolo-
gic activities, e.g., the former plant antitumor pro-
gram of the National Cancer Institute [1], often em-
ploys bioassays which cost more money than is avai-
lable for plant procurement and fractionation; in ad-
dition, the search for specific activities often over-
looks other useful activities which are not detected,
or are ignored, in the screening process. There is a
real need for reliable, general bioassays which can
detect a broad spectrum of pharmacologic activities
in higher plants and, yet, can be employed by natural
Universidad Contral de Venezuela, Caracas, Venezuela
* Visiting Assistant Professor from the Facultad de Farmacia,
product chemists, in-house at low cost, to guide phy-
tochemical screening and fractionation.
Since most active plant principles are toxic at ele-
vated doses, a possible approach to developing an ef-
fective general bioassay might be simply to screen for
substances that are toxic to zoologic systems, Once
such substances have been isolated, a battery of spe-
ific and more sophisticated bioassays could then be
employed. Desiring a rapid, inexpensive, in-house,
bioassay for screening and fractionation monitoring
of our physiologically active plant extracts, we have
been using a tiny crustacean, brine shrimp, as the ge-
neral bioassay tool.
The eggs of brine shrimp, Artemia salina Leacn,
are readily available at low cost in pet shops as a food
for tropical fish, and they remain viable for years in
the dry state. Upon being placed in a brine solution,
the eggs hatch within 48 hours, providing large num-
bers of larvae (nauplii). Brine shrimp have been pre-
viously utilized in various bioassay systems. Among
these applications have been the analysis of pesticide
residues [2-5], mycotoxins [6-11], stream pollu-
tants [12], anesthetics [13], dinoflagellate toxins [14],
morphine-like compounds [15], toxicity of oil disper-
sants [16], cocarcinogenicity of phorbol esters [17],
and toxicants in marine environments [18]. Most
workers have made use of the hatched nauplii, alt-
hough inhibition of hatching of the eggs has also been
studied [19]
In monitoring fractionation studies, brine shrimp
have previously shown utility with fungal extracts di-
rected at isolation of mycotoxins [20]. But for scree-
ning and fractionation of active materials from hig-
her plants, they have not been used. The organism is
now suggested as a convenient probe for pharmaco-
logic activities in plant extracts which may be manife-
sted as toxicity towards the newly hatched nauplii
Results and Discussion
We have developed a method whereby com-
pounds and extracts are tested at concentrations of,
82 Meyer ot al
Table | ‘We suggest that this simple bioassay system might
Brine Shrimp Bioassay Results forKnown Active NaturalProducts be readily utilized by pharmacognosists and natural
product chemists in the detection and isolation of
natural compound LOsinugint higher plant constituents with a variety of pharmaco-
logic activities. It has the advantages of being rapid
podophy/toxin 24 (24 hours following introduction of shrimp), inex-
eee aa a pensive, and simple (e.g., no aseptic techniques are
— acy required). It easily utilizes a large number of conti-
arene ties a nuously available organisms for statistical considera-
Scie vesetea z8 tions and requires no special equipment or training
stophenthin 218 and a relatively small amount of test sample (50 mg
arbutin 215 for crude extracts). Active compounds thus obtained
cattone 306, could then be subjected to more elaborate bioassays
thymat 514 for specific pharmacologic activities.
atropine SO, 686
santonin > 1000
10, 100, and 1000 jg/ml after being applied to filter
paper discs which are placed in vials containing brine
and ten shrimp in each of five replicates. Survivors
are counted after 24 hours and the percentage of de~
aths at each dose is recorded. These data can then be
used to estimate L's for comparison of potencies.
To explore the range of natural products which
could be detected in the bioassay, a number of
known active natural compounds were tested. These
results (Table 1) demonstrated the general utility of
the bioassay with compounds of diverse structures
and modes of activity. These results encouraged furt-
her testing of the assay for its use in screening and
eventual fractionation of active plant extracts.
‘As a demonstration project, the ethanol extracts
of seeds of 41 species of Euphorbiaceae were tested;
this plant family is well-known to contain toxic com-
pounds of diverse structures [21]. Data for the bioas-
say of these extracts are shown in Table IT; 9KB and
9PS cytotoxicities were determined and are shown
for comparison [22, 23].
Eighteen of the cuphorb extracts displayed to
ty (LCi < 1000 ug/ml) in the brine shrimp bioassay.
Several of these are known to contain physiologically
active principles (see Table II for references). Of 24
showed 9PS activity (LC) = 30 ug/ml),
to brine shrimp. The brine shrimp bio
assay is not specific for antitumor or, indeed, any
particular physiological action; but for a significant
number (14 of 24) of the species with cytotoxic activi-
ty, it may be possible to monitor fractionation using
principally the brine shrimp bioassay rather the more
expensive and time-consuming cytotoxic assays. Oc-
casional cross-monitoring in the more specific assay
systems would be necessary to ensure that the activi-
ties continue to correlate. The brine shrimp bioassay
is clearly a more general test than the 9KB assay in
which only six species were active (LCs) = 30 ug/ml)
(Table II); only two of these showed significant acti-
vity in the brine shrimp assay.
Experimental
Plant Materials
‘Samples of authenticated seeds of 41 species of Euphorbiaceae
were obtained from the seed collection of the Northern Regional
Research Laboratories of the United States Department of Agri-
calture in Peoria, Mlinois, U.S.A. All samples were weighed,
ground and defatted by shaking several times in hexane. The seeds
‘Wore then shaken several additional times with ethanol, and the
ethanol extracts were concentrated in vacuo and weighed.
‘All pure compounds employed were obtained from commer-
cial suppliers
Optotoxicty Testing
‘9KB and 9PS cytotoxiciies were determined inthe Purdue Cell
Culture Laboratory following protocols established by the Natio-
nal Cancer Institute [23]. Activities for extracts are considered s-
{nificant when LCs are = 30 gi
Sample Preparation
‘Samples were prepared by dissolving 0 mg of compound or ex
teact in Sm of methanol (Solution A) Solution B was prepared by
AiatingO-S ml of A to 10 ma with methanol. Appropriate amounts
ofsolution (10048, S0,IA, and S004 for 10, 100, and 1000 a!
tl respectively) were transferred to 1.25 om (1/2in) discs of filter
paper (Seueicnex and Scwuctt, no, 7404E). The discs were dred
Iai, placed in 2 dram vials, ad then died farther in vacuo for
One hour. Control diss were prepared using only methanol. Five
replicates were prepared foreach dose level
Hatching he Shrimp
‘Brine shrimp eggs (Living World, Metaframe Ine., Elmwood
ark, N. J.07407, U.S.A.) were hatched in a shallow rectangular
dish (22 « 32cm) filled with artificial sea water which was prepa-
‘red with a commercial silt mixture (Instant Oceans, Aquarium Sy-
stem, Ine.) and double-disilled water. A plastic divider with se-
veral/2.mm holes was clamped in the dish to make two unequal
compartments, The eps (ca 50 mg) were sprinkled into the larger
‘compartment which was darkened, while the smaller compart-
‘ment was illuminated, After 48 hours the phototropic nauplii were
collected by pipette from the lighted side, having been separated
by the divider from ther shells
Bioassay
“Ten shrimp were transferred to each sample vial using» 9in di
sposuble pipette (Scientific Products, diSPo pipettes), and art
‘ial sea water was added to make 5 ml. The naupliican be counted
‘macroscopically in the stem of the pipette against alighted back-
‘ground, A drop of dry yeast suspension (Red Star) (3 mg in S ml
frtficial sea water) was added as food to each vial. The vials were
‘maintained under illumination, Survivors were counted, withthe
Brine Shrimp Bioassay
Table It
Brine shrimp bioassay results of ethanolic extracts of soeds of Euphorbiaceae
Percentdeaths at 24hr (05% Refer.
Species 10 100° 1000 LCs, confidence 9PSLCs) 9KBLC;, encesto
gil ygiml ug/ml ugiml interval’ girl gil Com
ponents
1. Eremocarpus seigerus (Hoox) Bex. 92 78 100-24 (14-97)1.76x10% inact
2. Euphorbia amygdaloides Oe 007d 0-07) 68 inact, —
3. Croton tigi. 2 7898 = 8017-47), 1000 = 558 inact.
20. Euphorbia paralias 0 0 48 > 1000 7 20.2 inact
21. Euphorbia eriophora Boss. 0 4 48 > 1000 - 203 inact.
22. Trowia nudiforaL. 0 2 56 >1000 «= -- = agoxt0* 10%
23. Euphorbia heterophylla. 10-28 © 42> 1000 inact.
24. JatrophacurcasL 0 232 > 1000 9.0
25. Chidoscolus tepiquensis(Cosr.and 1a 23 = 40 > 1000 7A
Ga.) Linoa
28. Daphniphylum humile Maso. Oeeees0) 4 > 1000 inact, act
27. Mallotusphilipensis La.) Moca. -ARo. (OseeesOaeee toe 1000) inact inact
28. Chrozophora tnctoria (L.)A. Juss. Drees) 4 >1000 inact. inact
29. Sapium haematospermum Musu.-ARc. (Obes sO 1088 1000] 62.4 act
30. Jatropha gossypifoliaL. a) 0 > 1000 inact, inact
31. EuphorbiafatcataL. 0 0 ~~ 6 1000 inact. inact
82. Excoecaria bussel (Pax) Pax 0 0 16 > 1000 129 inact.
33. Bischotlajavanica Bune ar) 2 >1000 389 inact
‘34. Macaranga perakensis Hook 0 19 © 10 > 1000 78 inact.
35. Manihotisoloba Srave.sy a) 6 > 1000 4033
36. Cnidoscoluselastcus Lunoe. a) 0 > 1000 335312
37. Manihot woodieana Mua 8 4 B10 82 414
38. Sapium sebiferum(L.) Ross. 0 14 ©. >1000 082 inact
39. Aleurites moluccane(L.) Wixo. 4 10 © 16 > 1000 aye on
40. Euphorbiamedicaginea Bais, fpeeees0) 0 > 1000 40, 599
41. Euphorbiamyrsinies . Oty ar inact, inact
* Where data were insuficient or probit analysis, LCs», were estimated using logit iransformation (s9@ text) which does not provide con
fidence interval.
sid of a3 x magnifying glass, after 6 and 24hours, and the percent
‘deaths at each dose and control were determined, The 24 hours
‘counts were more useful. In cases where control deaths occurred,
the data were corrected using Abbotts formula [32] % deaths =
[(test—controlicontrol] 100.
Cp Determinations
Eca's and 95 % confidence intervals were determined from
‘the 24 hour counts using the probit analysis method described by
Fivwey [33]. In cases where data were insufficient for ths techni-
que, the dose-response data were transformed into a straight line
by meansof a logit transformation [34]; the LD, was derived from
the best fit line obtained by linear regression analysis
34
Meyer et al
Acknowledgements
[BNM acknowledges support as the AFPE Charles J. Lynn Me-
‘moral Fellow. Thanks are expressed toC. R. Surm.Jr.,and R. G.
Powe, USDA, Peoria, for providing the seeds. This work was
supported in part, by BMRG no, SOT-RR-OSS86 from the Natio=
nal Institutes of Health. NRF acknowledges a Research Scholars-
hip from Consejo Desarrollo Cientifico y Humanistico, Universi=
dad Central de Venezuela.
References
(2) Sutinss, Mand J. Douro, in
DeVita, VT. and H Busch (es)
Methods in Cancer Research, vl
XVI Cancer Drag Development,
Part Ap. 73, New York, 197,
‘Academie Press
(@) Michael, A"S.,C.G. Thomp.
fon and M.’ Abramovite” Science,
mol. 51,781 (198).
() Arcekal, Sand RP. H
‘wood: I Age: Food Chem. 8,32
(oe),
(5) Grossh,D.S:Science 155, 592
(969)
(6) Brows, R.F.,J.D. Wildman
fand RM. Eppley: J. Assoc. OF,
‘Anal, Chem 31,908 (1988).
@) Brown, RF: J. Am. Ol
(Chem, Soe. 46,119 (1969).
(8) Harwg, J. and P.M, Soot
Applied Microbiology 2/, 101
ssn,
(0) Eppley, R. MJ. Assoc. Of
‘Anal Chem. 97,618 (1979).
(G0) Korpinen, EL: Acta Path
Microbiol Seand., Sect. B 82, 465
(1939)
(i) Eng-Wilmot, D. and D. F.
Margins J. arm. Ss 68, 963
09%),
(02) Hood, D. W., T. W. Duke
tnd B, Stevenson I. Water Poll
Control Fel 32 864 (1960)
(3) Robinson, A-B., KF. Man-
Jy.M.P, Anthony, J.P. Catchpool
and L. Pauling: Science 1, 1255
965),
(18) Granade, H.R. C. Cheng
land N.J. Doorenbos: Pharm
$5. 65, 1414 0996),
(5) Richer, A. and A. Gold
Stein: "“Psychopharmacologia 17,
S27 (97).
(15) Ziliows, E.1., H.R. Foul
(C-Prager and J A" Cardin: J. We
ter Pollt. Contol Fed 5, 2389
(97,
(G2) Kinghorn, A-D., K.K. Har
Jesand NJ. Doorenbos: 3. Pharm.
53,6, 1368 (1967.
(8). Vanhaecke, PG. Persoone,
(C-Chaus, and P. Sorseloes, Fete
col. Environ, Safety 3, 382
(38.
(9) Chanh, PHL, and G. Many:
‘Aaresologc 4,595 (1983).
(20) Eppley, RUM. and W. 3. Bai
ley: Soenee #87, 758 (197)
Gt) Kingho," A. D., In King
for, AD. (ed), Toxic Plants. p
137, New York, 192, Columbia
University Pres
(2) Fergni, NR. JE, Putnam,
B. Anderson, LB. Jacobsen, and
J. LMeLaughli: J. Nat. Prod.
‘Gubsmitted for puiation,
(03) Goran, RL, NH. Groom
berg. MN. MacDonald, A.M
Schumacker and BI. Abbot:
Cancer Chemother eps pet, 3
1097),
(24) Farnsworth, NR, RN,
Blomster, W. M. Messmer. J.
King. GJ. Perinos and) 1D, Wie
es Loyaia 32,1 0960)
(25) Emmet, M4. W.: Proc. Am.
Tung Oi Asoc. 3 (1945).
(26) Clacer, Gand B. Tia: Bol,
Sedute Accad, Gioenia Sa. Nat.
(Catania 17, 108 (197).
(21) Obigashi H. and Mit
Bull. Inst Chem. Res, Kyoto
Univ. 50,239 1972).
(25) Hasibaan, V1. Res. Indian
Med. 9,70 (1973)
() Powell, R. G., D. Weised
snd CR Smithy des I. Org.
‘Chem, in pres
(G0) Kapehan, 8. MC. W. si
fel, MoI Mat, C.J. Gilmore and
RE Bryan: J Am. Chom Soe-08,
12295 (19765).
G1) Mourgue, M., J. Delephaut,
IR Baretand K. Kassab Bul Soo
Chim, Biol 43.517 (196).
(62)"Abboig, W. 8. Eson, Emto-
‘mol 18,255 (1925).
(G3) Finney, D. 1.2 Probie Analy-
i, Sede Cambridge University
Press, Cambridge, 1971
(G9 Hatter, EF. Heiner and.
Nogek: Arent Forsh. 27,1871
an,
Addayess: Prof. J. L. MeLaughlin,
Schoo! of Pharmacy and Pharmacal Sciences,
Purdue University, West Lafayete,
Tiana 47907, U.S.A.
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5819)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1092)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (845)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (540)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (348)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (822)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Herpes Zoster in The Patient With Hypertensive Crisis: A Case Report and Review of The LiteratureDocument1 pageHerpes Zoster in The Patient With Hypertensive Crisis: A Case Report and Review of The LiteraturePrayogi KramyNo ratings yet
- Urbanization and Urban Development PatternsDocument3 pagesUrbanization and Urban Development PatternsPrayogi KramyNo ratings yet
- Menstrual DisorderDocument36 pagesMenstrual DisorderPrayogi KramyNo ratings yet
- Massa PleuraDocument4 pagesMassa PleuraPrayogi KramyNo ratings yet
- Anatomi 3Document1 pageAnatomi 3Prayogi KramyNo ratings yet
- Anatomi 2Document1 pageAnatomi 2Prayogi KramyNo ratings yet
- Teaching Vocabulary Using Shared Reading and FlashcardsDocument6 pagesTeaching Vocabulary Using Shared Reading and FlashcardsPrayogi KramyNo ratings yet