Professional Documents
Culture Documents
11 36 1 PB
Uploaded by
oskrSSOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
11 36 1 PB
Uploaded by
oskrSSCopyright:
Available Formats
ASPEN HYSYS SIMULATION AND
COMPARISON BETWEEN ORGANIC
SOLVENTS (SULFOLANE AND DMSO) USED
FOR BENZENE EXTRACTION
*T. Zaiz, **H.Lanez, B.Kechida
Department of Process engineering, P.O. Box 789, 39000, El-Oued, Algeria
University of El-Oued., Algeria
ABSTRACT
Due to the high increase of the production of aromatic hydrocarbons: benzene, toluene and xylenes
BTX from oil because of the large activity of their big markets especially with the availability of
great quantities of these aromatic fractions in the oil. This study has two main parts the first
presents a general vision of the aromatic hydrocarbons, the second is going to focus on the liquidliquid extraction with the selected solvents as a separation method. The solvent selection depends
on many properties. In this second part there will be a simulation (conception and execution) of the
liquid-liquid extraction of aromatics by two different organic solvents Sulfolane and DMSO
followed by a comparison between the results obtained by the simulation. The simulator used will
be ASPEN HYSYS 7.2.
The results of the simulation showed that the use DMSO is better than Sulfolane because of the
separation efficiency the economic value and the regeneration rate although its use is more
dangerous (more toxic) than the Sulfolane
Keywords: Extraction, Aromatic, DMSO, Sulfolane, Simulation, HYSYS.
2013 IJCPS. All rights reserved
1. INTRODUCTION
The
petroleum industry has implemented several methods for separating
aromatic either cutting lubricant or light petroleum cuts, among these methods
there is liquid-liquid extraction. The liquid-liquid extraction is a separation
technique that takes advantage of the deference solubility of components of a
homogeneous liquid load in a suitable solvent [1, 4].
The addition to the load of a partially miscible solvent causes the appearance of a
second liquid phase which selectively transfers to the most soluble components.
Phase separation followed by decanting the solvent contained therein gives two
functions whose compositions depend on the parameters of the extraction [3,10].
It uses solvent extraction when distillation alone cannot provide a good separation
and good economic solution.
* Corresponding author. Tel.: +213 55 333 4904
E-mail addresses: zaizto@gmail.com (T. Zaiz), lanezhafnaoui@gmail.com (H.Lanez).
2013 IJCPS. All rights reserved.
T.Zaiz ett al. Internatiional Journall of Chemical and Petroleu
um Sciences, 22013, 2(1), 10
0-19
In ordeer to have a good exttraction, it is necessarry to use soolvent conttains the
followiing features:
- Solveent power.
- Selectivity. (Soluubility, seleectivity, den
nsity, viscossity, temperrature, it sh
hould not
be expeensive, toxic, corrosivee, unstable ... etc...)[11]].
2. OVE
ERVIEW OF
O AROMA
ATIC HYD
DROCARB
BONS
2.1 Chemical stru
ucture
Aromattic are diffferent than other hydrrocarbons by
b the fact that they have an
aromatic cycles. And
A C/H ratiio is high.
Benzenne has the formula
fo
C6H6, H are all identical since
s
the suubstitution of
o one of
the six hydrogen is
i a radical provided by
y a single compound.
c
The molecu
ule must
therefoore be symm
metrical. Thhe first form
mula of bennzene by Keekule was proposed
p
[1]:
Fig.1.Benzene formula..
It makees much off the fact that
t
all hyd
drogens aree identical, and there is not a
single derivative
d
substituted with
w a group
p (Y)
Fig.2.Tolueene formula..
For Xyylene three isomers
i
couuld exist
Orthho Xylene
Meta Xylene
Paraa Xylene
Tab
ble.1.Characteristics of aromatic com
mpounds [2]
form
mula
benzzene
Toluuene
o-xyylene
m-xyylene
p-xyylene
C6H66
C7H88
C8H110
C8H110
C8H110
MW
Boiling
Point (C))
Melting
Point (C))
density
(g/ml)
Solubility iin
water (g/1000g)
Dieleectric
Consta
ant 3,4
fl
flash
poin
nt (C)
78.11
92.14
106.177
106.177
106.177
80.1
110.6
144
139.1
138.4
5.5
-93
-25.2
-47.8
13.3
0.879
0.867
0.897
0.868
0.861
0.18
0.05
Insolublee
Insolublee
Insolublee
2.2
28
2.38(25)
2.5
57
2.3
37
2.2
27
-11
4
32
27
27
11
T.Zaiz et al. International Journal of Chemical and Petroleum Sciences, 2013, 2(1), 10-19
2.2 Different methods of separation of aromatic
We can use several techniques to extract high purity aromatics species produced
either in the steam cracking, or catalytic reforming.
These treatments are mostly based on physico-chemical processes and are
sometimes Sir most specific economic, certain types of load or certain operating
conditions although they are, in principle, able to handle all aromatic types
essences. They are: Crystallization, adsorption, distillation, azeotropic distillation,
extractive distillation and solvent extraction [5, 7].
3. EXTRACTION BYSOLVENT
Liquid-liquid extraction (also called solvent extraction) was initially utilized in the
petroleum industry beginning in the 1930s. It has since been utilized in numerous
applications including petroleum, hydrometallurgical, pharmaceutical, and nuclear
industries. Liquid-liquid extraction describes a method for separating components
of a solution by utilizing an unequal distribution of the components between two
immiscible liquid phases. In most cases, this process is carried out by intimately
mixing the two immiscible phases, allowing for the selective transfer of solute(s)
from one phase to the other, then allowing the two phases to separate[8,9].
Typically, one phase will be an aqueous solution, usually containing the
components to be separated, and the other phase will be an organic solvent, which
has a high affinity for some specific components of the solution.
The process is reversible by contacting the solvent loaded with solute(s) with
another immiscible phase that has a higher affinity for the solute than the organic
phase. The transfer of solute from one phase into the solvent phase is referred to
as extraction and the transfer of the solute from the solvent back to the second
(aqueous) phase is referred to as back-extraction or stripping [4].
The two immiscible fluids must be capable of rapidly separating after being mixed
together, and this is primarily a function of the difference in densities between the
two phases. [3, 6]
3.1 Different types of common solvents
The solvents used in industrial processes are either oxygenated sulfur compounds
such as tetra ethylene sulfone (sulfolane) or dimethyl derivative. They obviously
have the general properties of industrial solvents [7].
Fig.3.Sulfolane and DMSO structures.
12
T.Zaiz et al. International Journal of Chemical and Petroleum Sciences, 2013, 2(1), 10-19
3.2 Physico-chemical properties
Table.2.Physico-chemical properties of common solvents.
Solvent
M(g/mol)
Tf(C)
Tb(C)
20 C
Sulfolane
120.2
27.6
287
1266
10.3/30
DMSO
78.13
18.5
190.85
1100
1.99/25
Pressure of
steam (kPa)
-3
1.3310 30C
0.084 25C
4. ENVIRONMENTAL PROPERTIES
4.1 Toxicological information
Table.3.Toxicological information of common solvents.
Solvent
Toxicityague LD50
Risk indication
Values
carcinogenesis
Sulfolane
(Oral)
1941
(skin)
4009
R22
0.37
Negative results
DMSO
28000
50000
R36, 37,38
50
Negative results
R36/37/38: Irritating to eyes, respiratory system and skin.
R22: Harmful if swallowed.
4.2 Ecological information
The organic solvents mentioned are all volatile organic compounds (VOCs).Their
vaporization in the atmosphere contributes to the production of ozone in the
troposphere by photo chemical reaction, thus increasing the risks, especially for
asthmatics or people with breathing difficulties.
The rejection of these solvents directly into the environment can contribute
significantly to the deterioration of the flora and fauna inhabiting the rivers and
streams.
5. THE SIMULATION STEPS
5.1 General methodology
This part is to simulate an aromatics extraction process by ordinary solvents
previously presented on an industrial scale by using simulation software well
known in the engineering field as ASPEN HYSYS 7.2.
5.2 Choosing a-separation system
The benzene extraction of benzene / heptane mixture by ordinary solvents was
chosen as a model for aromatic / aliphatic separation.
5.3 Choice of a thermodynamic model
Data interaction parameters for the system heptane + benzene + solvent were
estimated by choosing the thermodynamic model NRTL (Non Random Two
Liquid), because it is the most suitable model for the simulation of liquid-liquid
13
T.Zaiz et al. International Journal of Chemical and Petroleum Sciences, 2013, 2(1), 10-19
extraction processes.
5.4 The operating conditions
The average conditions of ordinary solvents studied in the extractor are
determined according to existing industrial processes.
Table.4.Operatingconditions of the extraction processes of aromatic industrial scale.
Process
Solventused
Operating
Conditions
Report
solvent/feedstock
Number
of stages
Sulfolane
(Shell-Uop)
Sulfolane
100C
2bar
4/1
12
IFP
DMSO
35C
1bar
4/1
12
Table.5.Data of the extraction by organic solvents process.
Temperature C
Pressure bar
Total flow total t/h
Benzene t/h
Heptane t/h
Solvent t/h
Benzene
Heptane
Solvent
Feed
Solvent
Shell-UOP IFP
Shell-UOP
100
35
100
1.013
1.013
1.013
300
300
1600
120
120
180
180
1600
Composition by weight%
40
40
60
60
100
IFP
35
1.013
1600
1600
100
6. PROCESS DESCRIPTION
The feed mixture is fed to the liquid-liquid extraction tower (T-100), where
aromatic hydrocarbons selectively dissolved with the solvent. The raffinate phase
rich non-aromatic hydrocarbons out the top of the column and sent to the tower
(T-102) for the purpose of recovering the small amount of solvent introduced into
the raffinate phase. The water-solvent mixture passed to the stripper for
separation. Aliphatic out the top of the splitter and sent to storage. The solventrich extract phase exits the extraction tower with traces of aliphatic and aromatic
high quantity, they are sent to the stripper (101-T) to ensure good separation
solvent / aromatic solvent leaves the pure stripper (T-101) at the bottom and
recycled to the extraction tower and aromatics lot with high purity as distillate.
14
T.Zaiz et al. International Journal of Chemical and Petroleum Sciences, 2013, 2(1), 10-19
Fig.4.Technological scheme of aromatic extraction by organic solvent designed by
AspenHysys7.2.
15
T.Zaiz et al. International Journal of Chemical and Petroleum Sciences, 2013, 2(1), 10-19
Table.6.Results of the different processes studied.
Alimentation
processes
Temperature C
Pressurebar
Total flowt/h
Benzenet/h
Heptanet/h
Solventt/h
H2O
1
100
1.013
300
180
120
-
2
35
1.103
300
180
120
-
Benzene
Heptane
Solvent
H2O
60
40
60
40
Benzene
Heptane
Solvent
H2O
Solvent
1
100
1.103
1206
7.71
1186.22
12.06
2
33.36
1
1520
1289.67
230.32
Composition by weight %
0.93
92.77
84.85
6.29
15.15
Recovery rate %
-
Bottoms
Extracted Product
1
129
3
1412
253.86
36.13
1050.52
71.49
2
36.16
1
1737
162.08
26.35
1013.86
234.70
1
98
3
93.45
2.93
86.23
4.19
0.08
2
-5.89
1
82.94
17.98
2.56
74.4
5.06
9.33
1.52
75.64
13.51
3.14
92.28
4.49
0.09
0
99.24
0.73
0.03
0.01
98.96
0.04
99.82
0.18
82.31
0.60
0.02
100
100
. 1- Extraction by sulfolane 2- Extraction by DMSO
16
T.Zaiz et al. International Journal of Chemical and Petroleum Sciences, 2013, 2(1), 10-19
7. RESULTS AND COMPARISON
Following the processes carried out and which were designed to compare the rate
of solvent recovery without reducing the performance of processes it has been
found that there is a difference between these two.
On sulfolane, it was found that the extract contains 253 .86 t\h Benzene, while
DMSO contains only 162 .08 t\h (91.78 t\h more). The amount of benzene lost in
the raffinate is negligible (<3 t\h).
Pure benzene recovered was 184.19 t\h after extraction by sulfolane and 185.31
t\h by the DMSO.
Heptane for the amount in the extract was 36.1 t\h for sulfolane extraction and
26.35t\h for DMSO (difference 9.78 t\h) the major amount of heptane was in the
raffinate as in sulfolane extraction processes the amount of heptane was 86.23t\h
and 82.31 for DMSO (3.92 t\h difference).
The pure heptane to recover the end of the process is: 85.38 t \ h after extraction
sulfolane and 113.2 t \ h after extraction by DMSO.
According to that introduced 300 t\h mixing (180 t\h benzene and 120 t\h heptane)
in the extraction column was recovered 269.57 t\h in sulfolane extraction
(89.85%) and 298.51t\h by the method of DMSO (99.50%).
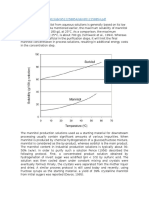
benzne/heptane productivity %
100
95
90
85
80
75
70
sulfolane
DMSO
Fig.5.Products obtained at the end of the process.
17
T.Zaiz et al. International Journal of Chemical and Petroleum Sciences, 2013, 2(1), 10-19
7.1 The solvents
a- Regeneration rate
Sulfolane introduced into the extraction column is 1186.22 t\his recovered solvent
is 1179.84t\h (99.31% sulfolane was recovered).
DMSO introduced into the extraction column is 1289.67 t\h and recovered DMSO
is 1185.12 t\h (99.90% solvent was recovered).
b- Economic comparison
Proposed by the company of Liaoyang Guanghua Chemical Co.., Ltd. Standard
sulfolane price in the market is 3700 $ per ton. In the process we will use 1206 ton
of sulfolane that reach up to 4,462,200 $.
99.31% of sulfolane was recovered means that there is a lost value of 30,789.18$
per hour.
The reasonable price of DMSO is about 2100$ for ton (2.1$ / kg) so as proposed
by Hansen Zhuzhou Chemical Co., Ltd. 1520 ton will be used with the value of
3,192,000$.
99.90% DMSO was recovered means that the lost value of this solvent is 3.192$
per hour.
While DMSO is cheaper than sulfolane as the total difference is 1,270,200$ and
the difference of the lost (per hour) is 27,597.18$.
8. CONCLUSION
We have seen in this work the importance of separation processes and took the
liquid-liquid extraction as a separation method in industry and its economic
importance.
For benzene in the aromatic hydrocarbons, liquid-liquid extraction is probably the
best method of separation and the solvents used are generally organic.
The preparation and the choice of solvent is probably the main processes such as
extraction is chosen such as to form with the support a mixture of two immiscible
phases and must not only allow the separation of the products but also be easily
used in extractors and easily separable from the dissolved products and its use
should be as economical as possible.
In this work we used two organic solvent which are: tetramethylene sulfone
(sulfolane) and dimethyl sulfoxide (DMSO) and compared the results of these last
two in the industry by simulation (with HYSYS 7.2).
We used the thermodynamic model NRTL (Non Random Two Liquid), because it
is the most suitable for the simulation of liquid-liquid extraction processes model.
The procedures were similar for both solvents means that the same equipment was
used for the separation and recovery same conditions operating power but
operating conditions solvents were not the same after the nature of the two
different solvents.
18
T.Zaiz et al. International Journal of Chemical and Petroleum Sciences, 2013, 2(1), 10-19
From the results obtained it was found that DMSO was better than sulfolane for
different reasons as it has better selectivity capacity miscibility regeneration rate
and economic value although it is more toxic than sulfolane.
9. REFERENCES
[1]
[2]
[3]
[4]
[5]
[6]
[7]
[8]
[9]
[10]
[11]
Extraction des aromatiques: Calcul de vrification de la colonne de
Benzne de
l'unit 200/RA1/K Ould Mohamed Lemine Beyrouk
Reziouk Faris.
Vogel's Textbook of Practical Organic Chemistry 5th Ed. (Furnis B.S. Et
All, Longman 1989).
Liquid-Liquid Extraction Equipment Jack D. Law and Terry A. Todd
Idaho National Laboratory.
Etude de lutilisation des liquides ioniques comme cosolvants pour
lextraction des composs aromatiques lchelle industrielle BENNOUH
Oussama.
P. WUITHIER : Raffinage et gnie chimique, Tome I,2eme dition 1972
Paris.
P. WUITHIER : Raffinage et gnie chimique, Tome II,2eme dition 1972
Paris.
J.P.WAUQUIER : Procds de sparation, Editions TECHNIP 1998
Paris.
Engineering PERRY HAND BOOK, Edition 1972.
Engineering DATA BOOK NINTH, Edition 1972.
Manuel opratoire de lunit GPL et celui de lunit de traitement de
RHOURDE-NOUSS.
R. ABDOULLAEV, V. KOSSIAKOV : Thorie et calcul de la
rectification des mlanges complexes,Editions INHC 1977 Boumerdes.
19
You might also like
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- 122 Lab Sample Report Rev 2Document7 pages122 Lab Sample Report Rev 2karenNo ratings yet
- CrystallizationDocument2 pagesCrystallizationoskrSSNo ratings yet
- Extraction of Eugenol From ClovesDocument12 pagesExtraction of Eugenol From ClovesmarcelompassosNo ratings yet
- Essential Oil Report SheetDocument1 pageEssential Oil Report SheetoskrSSNo ratings yet
- Extraction of Eugenol From ClovesDocument12 pagesExtraction of Eugenol From ClovesmarcelompassosNo ratings yet
- FC 0614 EmersonPortal OBanionDocument6 pagesFC 0614 EmersonPortal OBanionoskrSSNo ratings yet
- Extraction of Eugenol From ClovesDocument12 pagesExtraction of Eugenol From ClovesmarcelompassosNo ratings yet
- Simulation of Sulfuric Acid Plant Using Aspen-HYSYSDocument3 pagesSimulation of Sulfuric Acid Plant Using Aspen-HYSYSacckypenrynNo ratings yet
- Function: %KJ/KG %KJ/KG K %KJ/KG K %KDocument2 pagesFunction: %KJ/KG %KJ/KG K %KJ/KG K %KoskrSSNo ratings yet
- Vol 26 (3) BDocument7 pagesVol 26 (3) BoskrSSNo ratings yet
- Experiment On Packed Bed Column - Mass TransferDocument15 pagesExperiment On Packed Bed Column - Mass TransferAllyana Marie TiemsimNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (894)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Exercise 1: Chọn đáp án đúng:: Buổi 9: Động từ khuyết thiếu (Modal verb)Document6 pagesExercise 1: Chọn đáp án đúng:: Buổi 9: Động từ khuyết thiếu (Modal verb)Huyền HồNo ratings yet
- Aling Presing ChichacornDocument2 pagesAling Presing ChichacornMhel Joshua Bautista HermitanioNo ratings yet
- Cracking The SQL InterviewDocument52 pagesCracking The SQL InterviewRedouan AFLISSNo ratings yet
- A Very Rare Type of NeuralgiaDocument2 pagesA Very Rare Type of NeuralgiarosaNo ratings yet
- Labcir - Marwa - FinalDocument119 pagesLabcir - Marwa - FinalMashavia AhmadNo ratings yet
- Research Trends On Environmental Energy and Vulnerability - 2021 - Energy andDocument27 pagesResearch Trends On Environmental Energy and Vulnerability - 2021 - Energy andCynthia Mac-beathNo ratings yet
- Robert K Boscarato and Matthew Skaggs Corprate Credit Book Draft 1.Document70 pagesRobert K Boscarato and Matthew Skaggs Corprate Credit Book Draft 1.Robert BoscaratoNo ratings yet
- Gray Iron Castings: Standard Specification ForDocument6 pagesGray Iron Castings: Standard Specification Forsafak kahramanNo ratings yet
- T e 2552674 Percy Polls Peculiar Plants Fiction Year 5 Reading Comprehension - Ver - 5Document20 pagesT e 2552674 Percy Polls Peculiar Plants Fiction Year 5 Reading Comprehension - Ver - 5mariam osamaNo ratings yet
- Chen2019 PDFDocument5 pagesChen2019 PDFPriya VeerNo ratings yet
- Acoustic Emission InspectionDocument7 pagesAcoustic Emission InspectionAntonio PerezNo ratings yet
- Science Grade 7Document8 pagesScience Grade 7Lacus ClyneNo ratings yet
- Main Body Recruitment Process of Human Resource Division in Brac BankDocument55 pagesMain Body Recruitment Process of Human Resource Division in Brac BankAsfia PrantyNo ratings yet
- 3M SS Filters Data SheetDocument4 pages3M SS Filters Data SheetbinnisfquoteNo ratings yet
- 3 Payroll ReportDocument4 pages3 Payroll ReportBen NgNo ratings yet
- Role of Various Avonoids: Hypotheses On Novel Approach To Treat DiabetesDocument6 pagesRole of Various Avonoids: Hypotheses On Novel Approach To Treat DiabetesYuliet SusantoNo ratings yet
- OnScreen B2 Quiz 2ADocument2 pagesOnScreen B2 Quiz 2ALaura B.100% (1)
- Williamstown Cannabis Cultivation PlanDocument48 pagesWilliamstown Cannabis Cultivation PlanOltion JaupajNo ratings yet
- Understanding Income Statements EPS CalculationsDocument39 pagesUnderstanding Income Statements EPS CalculationsKeshav KaplushNo ratings yet
- Kristine Jane T. Zipagan Assignment: 1. Parts of InfographicsDocument2 pagesKristine Jane T. Zipagan Assignment: 1. Parts of InfographicsChristyNo ratings yet
- Fundamentals Deep Learning Activation Functions When To Use ThemDocument15 pagesFundamentals Deep Learning Activation Functions When To Use ThemfaisalNo ratings yet
- African in The Modern WorldDocument18 pagesAfrican in The Modern WorldSally AnkomaahNo ratings yet
- Solution SellingDocument18 pagesSolution Sellingvikramgulati13090% (1)
- Micro800 4-Channel and 8-Channel Analog Voltage/Current Input and Output ModulesDocument12 pagesMicro800 4-Channel and 8-Channel Analog Voltage/Current Input and Output ModulesSyarifudin WahidNo ratings yet
- Desantis, AlanDocument18 pagesDesantis, AlanOreillerNo ratings yet
- (Food Engineering Series) Gustavo V Barbosa-Cánovas - Humberto Vega-Mercado - Dehydration of Foods PDFDocument339 pages(Food Engineering Series) Gustavo V Barbosa-Cánovas - Humberto Vega-Mercado - Dehydration of Foods PDFLis FernandesNo ratings yet
- Teachers' Notes: ©film Education 1Document20 pagesTeachers' Notes: ©film Education 1רז ברקוNo ratings yet
- Hatsun Supplier Registration RequestDocument4 pagesHatsun Supplier Registration Requestsan dipNo ratings yet
- Document Revision TableDocument11 pagesDocument Revision Tableseva1969No ratings yet
- b1 Preliminary For Schools Classroom Posters and Activities PDFDocument13 pagesb1 Preliminary For Schools Classroom Posters and Activities PDFNat ShattNo ratings yet