Professional Documents
Culture Documents
Answer
Answer
Uploaded by
Alfie160 ratings0% found this document useful (0 votes)
98 views18 pagesanswer
Copyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentanswer
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
98 views18 pagesAnswer
Answer
Uploaded by
Alfie16answer
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You are on page 1of 18
Experiment 19
Separation and Qualitative Determination
of Cations and Anions
Introduction
Much of laboratory chemistry is focused on the question of how much of a given substance is contained in
‘a sample. Sometimes, however, the focus shifts to what substances are in the sample, rather than their quantity.
In this experiment, an analysis scheme for identifying both cations and anions in solution will be used in
determining the ions present in solutions containing unknown ions.
Concepts
© Qualitative analysis © Precipitation reactions
Background
Qualitative analysis is an analytical procedure in which the question “what is present?” is answered. In a
systematic qualitative analysis scheme, each substance present is separated from the other substances. Then a
confirmatory test is used to prove that the isolated substance is the expected one.
‘To begin the lab experiment, a solution containing six cations is analyzed using the techniques for
i qualitative analysis of cations. A second solution containing six anions is then analyzed using the qualitative
scheme for anions.
‘Two solutions, one containing any combination of six different cations, and another containing any
| combination of six anions, will be assigned to each student group. The two “unknown” solutions are then
analyzed to determine which ions are present and which are absent.
‘This experiment is carried out on a semi-micro scale. Very small quantities of reagents are used. Cleanliness
and a great deal of care are necessary to obtain good results.
While going through the steps of the analysis, keep a copy of the appropriate flow chart available for
reference. The flow charts appear at the end of the experimental directions. The charts will help to give the “total
picture” of where each analysis is and where itis heading, Read the directions carefully, and read the sections
which give the notes or chemical theory for each step. Don't just follow directions cookbook-style, but make an
effort to understand the chemical principles behind the procedures.
General Techniques for Qualitative Analysis
Keep Good Records
It is necessary to keep good records so as not to get confused and forget what solutions are in which test tubes.
‘Number the test tubes with pencil or permanent ink so the numbers do not come off in the hot water bath.
| ‘Maintain a current record of the work. Don’t trust the results to your memory. Below is an example from
step 1 of the cation analysis.
Known Solution Unknown Solution
Step Procedure Results Conclusion Results Conclusion
1 | Add HCI to known solution | White ppt forms. | Ag” present
in TTI. Centrifuge. Pour
the colored solution into
Tr
Note: TT is short for test tube. Fill out the columns for the “Known Solutions” first. Then, when analyzing
the “Unknown Solutions,” fill out the last two columns.
Laboratory Experiments for AP Chemistry 127
Experiment 19
Be Orderly
Arrange the chemical reagents in a way so that it is easy to find the solutions needed. Put acids together,
bases together, for example.
Avoid Contamination
‘Tap water is often a source of contaminating ions. Wash and rinse all glassware with distilled or deionized water,
A stirring rod is constantly used to mix solutions, and it also must be rinsed with distilled or deionized water
so that it does not contaminate subsequent solutions. An easy way to do this is to fill a 400-mL beaker about 46
full of distilled water, and keep your stirring rods in this beaker. The small amount of contaminants present in
this volume of water should cause no problem, Replace the distilled water as needed.
Droppers or plastic pipets should also be rinsed twice with distilled or deionized water after they are used.
Get in the habit of rinsing them immediately after use.
‘When you use chemical reagents in dropper bottles, do not turn the droppers upside down. This causes the
reagent to go into the rubber dropper top, which may dissolve the dropper top and contaminate your solution.
Measuring Solutions
Generally, the volume of solutions added during this lab should be estimated. It is not necessary to use a
graduated cylinder to measure solution volumes. A test tube can be calibrated in milliliters to give an idea of
what volume a milliliter actually is.
Heating Solutions
Frequently it will be necessary to heat a solution to speed up a reaction. Do NOT heat small test tubes over
Bunsen burner flames. A sudden steam bubble will cause the solution to shoot out of the test tube. Instead, heat
test tubes in a boiling water bath. A good idea is to set up the boiling water bath when beginning work in the lab
because it may take time to heat the water to boiling,
Stirring Solutions
Each time a reagent is added to a test tube, the solution needs to be stirred. It is important to mix the
solutions at the top and the bottom of the test tube. A stirring rod that is flattened at the bottom can be used as @
plunger to effectively mix solutions in narrow test tubes.
Separating Solids from Solutions
Mixtures must be centrifuged so that the solid will pack down at the bottom of the test tube and the solution
above it can be removed. Don’t forget to counterbalance the test tubes in the centrifuge with similar test tubes
holding equivalent volumes of liquid (Figure 1). Let the centrifuge spin for about 30 seconds. Usually the
supernatant liquid (the liquid above the precipitate) can be poured off of the precipitate. Sometimes precipitates
tend to float on the surface of the solution. If this is the case, use a Beral-type pipet to draw off the supernatant
liquid. Its better to leave alittle liquid over the precipitate than to transfer some of the precipitate.
+ Never fil centrifuge tubes to capacity. Keep quid levels at |
keep the rotor balanced, 2Tubes Tubes
| 6 tFonty one tube needs tobe centrifuged,
Fill ll tubes tothe same height. Gp &
inserting an additional tube (labeled asa “blank’) containing ae) +
« Follow manufacturer’ directions.
|___thesame volume of iid 2Tubes 3 Tubes Tubes 6 Tues
Figure 1, How to safely load and use a centrifuge.
ao Laboratory Experiments for AP Chemistry
Washing Precipitates
Itis almost always necessary to wash precipitates to free them from
ions that might cause confusion in later steps. To do this, add 1 or 2 ml.
of distilled or deionized water to the precipitate, stir, centrifuge, and
discard the wash water. Sometimes the directions will require a specific
reagent in the wash water.
Checking the pH
‘To check the pH ofa solution, put a piece of litmus paper or pH
Paper on a clean glass plate or watch glass. Dip the stirring rod into the
solution in the test tube, and touch the stirring rod to the paper (see
Figure 2). Do NOT dip the test paper into the test tube, This may cause
some of the indicator dye to dissolve in the solution, and the indicator
color may confuse subsequent tests.
Storing Solutions
To keep a solution until the next laboratory period, stopper the test
tube with a rubber stopper. Ifa precipitate is present, put a few drops of
distilled or deionized water on it before stoppering the test tube. Be sure
to record a list of substances that are present in each test tube, Do not
rely on memory!
Experimental Overview
Experiment 19
Moisten the tip ofa clean
stirring rod withthe solution
tobe tested
f Place a drop
of solution on|
the test paper.
When applicable, immediately
compare test paper color with
the color chart
Figure 2. Using pH test paper
In this experiment you will analyze a solution that can contain any combination of six different cations and.
a solution that can contain any combination of six different anions. First of all, you will analyze a cation and an
anion solution that are “known” to contain all of the ions to learn the techniques for the analysis, Then you will
analyze an “unknown” solutions to determine which ions are present and which are absent.
Pre-Lab Questions
Use the flow charts at the end of the experimental procedure to answer the following questions. In each
question, a testis carried out to determine the presence or absence of the ions that are listed. State if the test
results indicate if each possible ion is present, absent, or undetermined,
1. Test for Ags, Cuts, Fe
‘Some 6 M HCI! is added to a solution that may contain the three ions. A white precipitate forms,
Tons present Tons absent:
2. Test for Mn*, Fe™, and Cu®*
Ions undetermined:
Some 3% H,O, and 6 M NaOH are added to a pale blue solution. A dark precipitate forms, which totally
dissolves when 6 M H,S0, is added, Addition of 6 M NH, to the solution until i is basic results in a deep blue
solution containing a dark precipitate. The dark precipitate completely dissolves in H,S0,
Ions present
3, Test for Cut, Al*, and Zn?
Tons absent: ons undetermine:
Addition of 6 M NH, to a solution known to contain one or more of the above ions causes the formation of a
deep-blue colored solution containing a gelatinous precipitate.
Ions present: Ions absent:
Tons undetermine:
Laboratory Experiments for AP Chemistry 129
[ —
Experiment 19
4, Test for CO, and Cl
‘Some 6 M HCI is added to the solution that may contain
solution is heated.
ons present: ___
5, Test for Ch, Brand 1
‘The test solution is acidi
and the mixture is shaken. After the layers have separate
Ions absent:
Tons absent:
6. Test for Cr, $0,", CO", NO,, Br, and
Tons present:
1 of AgNO, causes no precipitate to form. Additior
tate to form.
Ions present: Ions absent:
Only one of each of the following pairs of reactants unde1
for the reaction that occurs.
7, NaCl{aq) + BaCl,(aq) >
Na,S0,(aq) + BaCl,(aq) >
8, NO;{aq) + OF(aq) + Alls) >
COM(aq) + OH(aq) + Alls) >
9, Krlag) + CHaq) >
Agi(ag) + CHaq) >
Materials
Chemicals
Cation solution, 5 mL
Silver nitrate, AgNO,, 0.2 M
Copper(l) nitrate, Cu(NO,),, 0.2
Zine nitrate, Zn(NO,),, 0.2.M
Anion solution, 5 mL
Potassium iodide, KI, 0.2 M
Sodium bromide, NaBr, 0.2 M
Iron
Mang
Sodit
Sodium carbonate, Na,CO,, 0.2 M Sodi
Test Solutions
Acetic acid, CH,COOH, 6 M, 10 mL. Bari
‘Aluminum, Al, granules Hyd
Aluminon, 0.1%, 5 mL
Ammonia, NH,, 6 M, 10 ml,
Barium chloride, BaCl,, 0.1 M, 10 mL
Hyd
Sodit
the above ions. Formation of bubbles is noted as the
Tons undetermined:
,4 and KMnO, is added until the solution remains pink. Some mineral oi is added
AgCl(s)
‘2. Add 8 drops of 6 M HCI to the solution to be analyzed. Stir. A white precipitate indicates that the Ag: ion
is present.
5, Centrifuge the solution and test to be sure that precipitation is complete by adding one more drop of 6 M
HCI. No additional precipitate should form. lf more precipitate does form, continue adding 6 M HCI until
precipitation is complete.
c. Centrifuge, decant (pour off) the liquid, and save the liquid in a second test tube for procedure #3.
Alternatively, use a Beral-type pipet to draw off the supernatant liquid and transfer it to another test tube.
d. Wash the precipitate by adding 1 mL distilled or deionized water and stir. Centrifuge and discard the wash
water. Save the precipitate for procedure #2.
2. Confirmation of Silver
‘When 6 M NH, is added to AgCl, the Ag’ ion forms a colorless complex ion and goes into solution:
AgCl(s) + 2NH,(aq) <2 Ag(NH,),*(aq) + Cl(aq)
[Addition of hydrochloric acid to the Ag(NH,)* complex ion breaks apart the ion. The NH, combines with H”
to form NH,*, and the Ag* ion recombines with the CI- ion to precipitate as white AgCI
Ag(NHL,),"(aq) + CH(aq) + 2H"(aq) @ AgCls) + 2NH (2a)
t
a. To the precipitate from procedure Id, which is AgCl, add 1 mL. 6 M NH.
6. Stir until the precipitate completely dissolves.
c. Add 15 drops of 6 M HCI to the solution. The solution will smoke and the reaction between the strong
acid and the base will give off heat whether or not silver is present. The test tube may get very warm.
d, Stir and test with pH indicator paper or litmus paper to be sure the solution is acidic. If it is not acidic,
add more HCI. The reappearance of the white AgCI precipitate in the acidic solution confirms the
presence of silver.
e. Dispose of the silver compound as directed by the instructor.
3. Separation of Manganese, Iron, and Copper from Aluminum and Zine
Tn basic solution, the amphoteric zinc and aluminum ions form colorless complex ions and remain |
in solution, while the hydroxides of all the other ions will precipitate. Adding hydrogen peroxide to the
basic solution will oxidize Mn® to Mn**. Manganese will precipitate as black MnO, and the other ions will
precipitate as hydroxides: rust colored Fe(OH),, and blue Cu(OH),. The reactions are as follows:
Mn’(ag) + 20FM(aq) + Ma(OH),(s)
Mn(OH),(s) + H,0,(aq) > MnO,(s) + H,0(1)
Fe(aq) + 30H-(aq) > Fe(OH),(s)
Cu(aq) + 20H-(aq) + Cu(OH),(s)
AP*(aq) + 40H-(aq) > AN(OH), (aq)
| Znt(aq) + 4OHaq) > Zn(OH) aa)
132 Laboratory Experiments for AP Chemistry
Experiment 19
4. To the solution saved from procedure 1c, add 10 drops of 3% hydrogen peroxide, H,0,. With stirring, add
6M sodium hydroxide, NaOH, until the solution is basic and then add 3 more drops.
Stir and place the test tube in a hot water bath for 3 minutes, The formation of a precipitate indicates the
presence of either copper, iron, manganese, or a combination of the three.
. Centrifuge the solution, and separate the clear solution from the solid. Save the clear solution, which may
| contain Zn(OH)2-and Al(OH), ions for procedure #9. |
4. Wash the precipitate with a mixture of 10 drops of 6 M NaOH and 10 drops of water.
e. Centrifuge and discard the wash water. Save the precipitate for procedure #4. |
4, Separate Manganese Ions from Iron and Copper Ions
Manganese(IV) oxide, MnO, does not dissolve well in acid or base unless it is oxidized or reduced. The
other hydroxides that are present will readily dissolve in acid solution.
Fe(OH),(s) + 3H"(aq) > Fe“(aq) + 3H,0(1)
Cu(OH),(s) + 2H*aq) > Cu(aq) + 2H,0(1)
4a. Add 5 drops of water to the precipitate from step 3e. Then add 6 M H,SO, dropwise until the solution is
acidic when tested with litmus paper (about 6 drops).
6, Centrifuge and separate the MnO, precipitate, which may be dark brown or black, from the supernatant |
liquid which may contain the yellow Fe and blue Cu ions.
¢. Save the solution for procedure #5. Wash the precipitate with water, centrifuge, and discard the wash
water.
d, Save the precipitate for procedure #7.
5. Separation of Iron from Copper; Confirmation of Copper
Aqueous ammonia added to a solution in which Cu® is present will cause the deep blue tetraammine
copper(Il) complex ion to form. The presence of this deep blue color confirms the presence of copper. At
| | the same time, the basic ammonia solution will precipitate the hydroxide of iron.
Cu (aq) + ANH,(aq) > Cu(NH,) (aq)
Fe'(aq) + 3OH'(aq) Fe(OH),(s)
‘An additional and very sensitive confirmatory test for copper is to precipitate the red-brown copper(I)
| hexacyanoferrate() [also called copper) ferrocyanige), Cu,{Fe(CN),|(3), from a Cu’ solution,
4. To the solution from step 4c, add 6 M aqueous NH, until the solution is basic to litmus, and then add 1 ml.
extra,
5. Centrifuge and separate the supernatant liquid from the precipitate. Save the precipitate for procedure
#6, The presence of the blue Cu(NHL,) ion is the confirmatory test for copper.
| Note: For an additional confirmatory test, to the solution containing the Cu(NH,)2* add 6 M acetic acid,
| CH,COOH, until the blue color fades and the solution becomes acidic. Then add 2 drops of 0.1 M
K,Fe(CN),]. A red-brown precipitate of Cu,{Fe(CN),] confirms the presence of copper.
cc. Dispose of the copper solution as directed by the instructor.
Laboratory Experiments for AP Chemistry
133
Experiment 19
Confirmation of Iron
Tron(Ill) hydroxide will dissolve in sulfuric acid. Addition of the thiocyanate ion, SCN’, forms a deep wine-
red colored complex ion with iron that is a very sensitive test for the presence of iron,
Fe(OH),(s) + 3H*(aq) — Fe'(aq) + 3H,0()
Fe(aq) + SCN-(aq) —+ FeSCN*(aq)
a, Wash the precipitate of iron hydroxide from procedure 56.
| +b. Add 6 M H,SO, dropwise until the precipitate dissolves.
c. To the solution add 5 drops of 0.1 M KSCN solution. The deep red FeSCN* ion confirms the presence of
iron.
4. Dispose of the iron solution as directed by the instructor.
7, Manganese Ions
‘Manganese(IV) oxide will dissolve in acid solution when a reducing agent is present. Notice that in acid
solution H,0, can act asa reducing agent. In procedure #3, however, H,O, in basi solution functioned as
an oxidizing agent.
Mn0,(s) + H,0,(aq) + 2H"(aq) > Mn®(aq) + 0,(@) + 2H,0())
a. To the precipitate from step 4d add 1 mL of water and 1 mL of 6 M H,S0,
5. Add 1 mL of 3% H,0, and heat in a boiling water bath with stirring, The precipitate should quickly
dissolve, leaving very little residue.
8. Confirmation of Manganese
In dilute HINO,, the bismuthate ion, BiO,, will oxidize Mn’* to the purple permanganate ion, Mn0,-. The
presence of this purple ion in solution is the confirmatory test for manganese.
2Mn*(aq) + 14H"(aq) + SBIO,(aq) > 2MnO-(aq) + SBP(aq) + 7H,O()
‘To the solution from step 7b add 1 mi of 6 M HINO, and add a spatula of solid sodium bismuthate,
NaBiO,. Some excess solid sodium bismuthate should remain, Add more if needed.
5, Stir and centrifuge to determine the color of the supernatant liquid. The purple color of the MnO, ion in
solution confirms the presence of manganese.
. Dispose of the manganese as directed by the instructor.
9. Separation of Zinc and Aluminum
[The colorless zinc and aluminum ions can be separated from one another because the aluminum forms an
insoluble hydroxide in ammonia solution, while the zinc forms a soluble complex ion with ammonia. ‘The
insoluble aluminum hydroxide is a translucent, gelatinous precipitate that is difficult to see.
Zn(OH),-(aq) + AH*(aq) > 2ni*(aq) + 4H,0()
Zn*(aq) + 4NH,(aq) > Zn(NH,)2*(20)
AH) (aq) + 4H"(aq) > AP*(aq) + 4H,0(0)
‘AP*(aq) + 3NHaq) + 3H,0(1) —> AI(OH),(s) + 3NH (aq)
4. To the colorless solution from step 3c add about 10 drops 6 M HNO,, Test to see ifthe solution is acidic.
b. Continue to add HNO, until the solution is acidic.
134 Laboratory Experiments for AP Chemistry
a
Experiment 19
© Then add 6 MINH, dropwise until the solution is basic to litmus
dt Add an additional 3 drops ofthe 6 M NH, Str the solution, A gelatinous precipitate of Al(OH), will be
present. It is difficult to see the precipitate,
e. Centrifuge, and carefully separate the supernatant liquid from the precipitate,
10. Confirmation of Aluminum
| The confirmation of aluminum is carried out by dissolving the ANOF, precipitate acl adding a red dye
called aluminon to the solution, and then reprecipitating the Al(OH),. The red dye will be adsorbed in the
Selatinous precipitate making the precipitate easier to see. This red gelatinous precipitate is called a “lake.”
AOH),(8) + 3H"(aq) > AP*(aq) + 3H,0()
AP*(aq) + 3NH,(aq) + 3H,0()) > AI(OH),(s) + 3NH,'(aq)
4@. Dissolve the precipitate from step $e in 6 M HCl.
6. Add 3 drops of aluminon solution, and then add 6 M NH, until the solution is basic to litmus, The red
aluminon should be adsorbed by the gelatinous Al(OH),
. Centrifuge so that it is easier to see if the aluminon is adsorbed by the precipitate rather than coloring the
solution red. The red precipitate confirms the presence of aluminum.
4. Dispose of the aluminum precipitate and solution as directed by the instructor
11. Confirmation of Zine
‘The confirmatory test for zinc is the formation of a precipitate of potassium zinc hexacyanoferrate(II),
| K.2n,{Fe(CN),|,. This precipitate is nearly white when pure, but ifa trace of iron is present, it may appear
light green or blue-green in color.
Zn(NH,) (aq) + 4H*(aq) > Zn*(aq) + NH,"(aq)
Szn(aq) + 2K-(ag) + 2Fe(CN),M (aq) > K,2n,{Fe(CN),),(s)
@. Make the solution from procedure 9e slightly acidic by adding 6 M HCI dropwise.
8. Add 3 drops of 0.1 M K,|Fe(CN),] and stir.
© Centrifuge to see the confirmatory precipitate of K,Zn,(Fe(CN),],, which will be white to light green or
blue green in color,
4d. Dispose of the zinc precipitate and solution as directed by the instructor,
12. Repeat steps 1-11 for the cation unknown sample. Be sure to record the results for each step.
Part 2. Qualitative Analysis of Anions
Note that the following directions are written for a “known” solution that contains all of the anions. An
“unknown” solution will probably not form all ofthe products described in this procedure. You should make
note of any differences as you analyze your “unknown” solution.
Aqueous solutions of all of the anions tobe tested are colorless. The positive ion associated with each of the
anions will be either sodium or potassium ion,
As with the analysis of cations, record the results of each step in the Anion Data Table.
Laboratory Experiments for AP Chemistry 135
Experiment 19
1. Separation of the Halides (CI, Br, I); Confirmation of Chloride
‘The halides all form insoluble silver compounds. Silver chloride is a white solid, silver bromide is a pale,
cream-colored solid, and the solid silver iodide is a light yellow solid.
Cag) + Ag‘(aa) > AgCI(s) |
Br(aq) + Ag'(aq) > AgBri(s)
Faq) + Ag‘(aq) > Asis)
Silver chloride is the only silver halide that dissolves in 6 M ammonia, NH,, forming the colorless jon
‘Ag(NH,),. If nitric acid, HNO, is added to a solution containing this ion, the ammonia in the complex
reacts with hydrogen ions to form ammonium ions, and the silver recombines with the chloride fons which.
are still present in solution,
es + 2NH,(aq) > Ag(NH,),"(aq) + Cr(aq)
Ag(NHL),"(aq) + CH(aq) + 2H'(aq) > AgCl(s) + 2NH,(aq) |
‘a. Place 10 drops of the original test solution (or unknown solution) in a test tube. Test to see if the solution
is acidic. Ifit is not, add 6 M acetic acid, CH,COOH, dropwise with stirring until the solution is acidic.
‘Add 10 drops of 0.1 M silver nitrate, AgNO,, A precipitate of AgCl, AgBr, and Agl will form.
. Centrifuge and pour off the supernatant liquid.
d, Wash the solid with 0.5 mL distilled water, centrifuge and discard the wash water.
ce. Add 0.5 mL. 6 M ammonia, NH,, to the precipitate. Stir to dissolve any AgCI.
£, Centrifuge, and pour the supernatant liquid into another test tube to test for chloride ion.
49. Discard the precipitate of AgBr and AgI in a container provided for disposal of waste solids.
h.
|. Add 1 mL 6 M nitric acid, HINO,, to the solution containing the dissolved silver chloride. The solution will
get hot and smoke from the reaction with the excess ammonia whether or not silver chloride is present
i. Test with litmus or pH paper to see ifthe solution is acidic. If tis not, add more HNO, until the solution
is acidic. The appearance of the white precipitate of AgC! in the acidic solution confirms the presence of
chloride
2, Separation and Confirmation of Bromide and Iodide
Tadd solution, iron(lit ion, Fe isa weak oxidizing agent capable of oxidizing the easly oxidized |
jodide ion to iodine. Bromide and other ions present will not interfere. The nonpolar iodine will
preferentially dissolve in nonpolar mineral oil, where it can be identified by its pink to violet color.
21-(ag) + 2Fe%(aq) > I,{aq) + 2Fe™
Br,, Other ions present will not interfere. The nonpolar bromine can be extracted into nonpolar mineral
oil where it can be identified by its characteristic yellow to brown color.
| 10Br-(aq) + 2MnO,-(aq) + 16H*(aq) > SBry(aq) + 2Mn*(aq) + 8H,0() |
| KMn0, is a stronger oxidizing agent than the iron(II) nitrate and will oxidize bromide, Br, to bromine,
‘a Place 10 drops of the original test solution (or unknown solution) in a test tube, Add 6 M HNO, dropwise
with stirring until the solution is acidic.
6, Add 1 mL. 0.6 M Fe(NO,), in 0.1 M HINO, solution and stir.
136 Laboratory Experiments for AP Chemistry
Experiment 19
¢. Add 1 ml of mineral oil, stopper the test tube with a cork stopper, and shake for 30 seconds. The presence
of a pale pink to purple color in the mineral oil layer (the top layer) due to dissolved iodine confirms the
presence of I-in the original solution.
d. Remove the mineral oil (top layer) with a capillary dropper and discard in the container provided for
waste solutions,
@. Repeat the extraction with fresh mineral oil until all the iodine is removed. Discard the mineral oil layer.
f Add 0.1 M KMnO, solution to the aqueous layer dropwise with stirring until the solution remains pink.
4g. Again add 1 mL mineral oil, cork and shake the test tube for 30 seconds. The presence of a yellow to
brown color in the mineral oil layer due to dissolved bromine confirms the presence of Br- in the original
solution. Discard the solution in the container provided.
3, Confirmation of Carbonate
| In acid solution, carbonate forms carbon dio:
effervescence. Carbon
When carbon dioxide gas is passed through a saturated solution of barium hydroxide, it readily forms a
precipitate of white barium carbonate.
CO*(aq) + 2H'(aq) > CO,(g) + H,0)
CO,(g) + Ba’*(aq) + 20H'(aq) + BaCO,(s) + H,0(l)
as and water, The carbon dioxide may be seen asa slight
-xide is less soluble in hot water than cold water.
If any bubbles were formed when acid was added to the original solution, carbonate is probably present
and carbon dioxide is being formed. A confirmation of the presence of carbonate involves reacting evolving
carbon dioxide with barium hydroxide to form white, insoluble barium carbonate.
4a. Place 2 mL of clear, saturated Ba(OH), solution in a test tube to be available for the test with carbon
dioxide.
6. Place 1 mL of the original test solution (or unknown sol
. Acidify this solution by adding 0.5 mL of 6 M HNO,.
nn) ina different test tube.
d, Place the tube in a hot water bath and observe to see if any gas bubbles form.
e. Take a dry Beral-type pipet and squeeze the bulb closed. Place the tip of the pipet close to (but not
| touching) the surface of the liquid in the test tube and slowly release the bulb to draw escaping carbon
dioxide into the pipet.
£ Put the pipet into the barium hydroxide solution, and slowly squeeze the bulb, causing the gas in the
pipet to bubble through the barium hydroxide solution. This procedure may be repeated, The formation
of a cloudy white precipitate of barium carbonate confirms the presence of carbonate ion in the original
j solution,
4, Confirmation of Sulfate
The test for sulfate is the formation of white, insoluble barium sulfate, This solid is insoluble even in acidic
solution.
SO} (aq) + Ba*(aq) —> BaSO,(s)
4, Place 0.5 mL of the original test solution (or unknown solution) in a test tube
5. Add 6 M nitric acid, HNO, dropwise until the solution is acidic.
¢, Add 0.5 mL 0.1 M BaCl, solution. The formation of a white precipitate of BaSO, confirms the presence of
sulfate.
| Laboratory Experiments for AP Chemistry 187
Experiment 19
5. Confirmation of Nitrate
[~The test for nitrate involves the reduction of nitrate ions in a basic solution to ammonia, NH, using solid
aluminum as the reducing agent. When the solution is heated, ammonia gas is liberated. The evolving
‘ammonia gas will turn litmus paper from pink to blue.
3NO,(aq) + 8Al(s) + SOH-(aq) + 18H,0(1) > 3NH,(@) + 8AI(OH) (aq)
a. Place 1 mL of the original test solution (or unknown solution) in a test tube,
6, Add 6 M NaOH dro
c. Use a Beral-type pipet to transfer the solution to the bottom of a dry test tube without getting the walls of
the test tube wet with solution.
with stirring until the solution is basic, and then add 6 drops in excess.
d. Add the tip of a spatula containing aluminum granules.
@. Place a small cotton wad loosely about halfway down the test tube, but not touching the solution. This is
to prevent spattering of the solution onto the litmus paper.
£, Hang piece of moist red litmus paper (or pH paper) in the tube so that the bottom of the paper is close
to (but not touching) the cotton.
4g. Warm the solution in a hot water bath until it starts bubbling strongly. Be sure that the solution and the
cotton do not touch the litmus paper.
hh. Allow the solution to cool. A slow color change (within 3 to 5 minutes) of the litmus paper from pink to
blue, starting at the bottom and spreading to the top, indicates the evolution of ammonia and confirms
the presence of nitrate ion in the original solution.
6. Repeat anion procedures 1-5 for the anion unknown sample.
Disposal and Cleanup
Your teacher will provide disposal and cleanup instructions.
138 Laboratory Experiments for AP Chemistry
Cation Analysis Data Table
Data Tables
Step Procedure Results
Conclusion
10 |
n
Laboratory Experiments for AP Chemistry
Experiment 19
___Unknown Solution
Results Conclusion
139
Experiment 19
Anion Analysis Data Table
Known Solution ‘Unknown Solution
Step Procedure Results Conclusion Results Conclusion
Post-Lab Questions (Use a separate sheet of paper to answer the following questions.)
1. What is the precipitating reagent for silver (Ag’)é Would a solution of NaCl work as well? Why or why not?
2, Inthe analysis scheme, Ag” is precipitated as AgCl, the precipitate is dissolved, and then AgCl is precipitated
again in the confirmatory step. Explain the chemistry of each of these steps by showing a balanced equation
for each.
3, When Fe** and Cu react with NH, solution they form two different types of products. One is a precipitate
and one is a complex ion in solution, Write equations for these two reactions.
4. H,0, can act either as an oxidizing agent or a reducing agent, depending on the acidity of the solution. Write
half-reactions for H,0, acting as an oxidizing agent and a reducing agent.
5. In separating Mn** from other ions, itis oxidized, reduced, and then oxidized. Write the equations for each
of the steps, and state the oxidation number of manganese in each product.
6. Explain why it is necessary to test for iodide by oxidation with Fe before the test for bromide by oxidation
with MnO, is done.
7. Write separate oxidation and reduction half-reactions for the procedure used in the test for nitrate ions.
8, In the nitrate test, why must care be taken to keep the moist litmus from coming in contact with the cotton
or the solution?
9. In step 4, Ba* is added to the solution containing all six ofthe anions and precipitates BaSO,, but not
BaCO,, However, in step 3, the precipitation of BaCO, is the confirmatory test for carbonate ion. Why doesn’t
BaCO, precipitate in step 4 but it does in step 32
140 Laboratory Experiments for AP Chemistry
Experiment 19
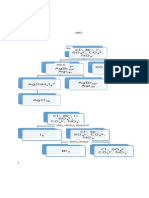
Qualitative Analysis of Cations
Flow Chart
hate Min) eRe (Cue Abs ca
1| HCl
| _Agcis) Mn Fee Cut ABE ne
2| NH,
— t
AatNH),” | See Next Page
HCL
Note: Each branch in the flow chart indicates a separation,
Solids are listed to the left
Boldface type indicates that a solid or liquid is present.
Ions in regular weight type are all present in aqueous solution.
‘Substances shown in heavy rule boxes are confirmatory substances.
Laboratory Experiments for AP Chemistry 771
Experiment 19
Mn Fe
__
cu
3 HO,
NaOH
AP an
Cations Flow Chart, continued
L
Mn0,(s) Fe(OH),(s) _ Cu(OH),(s) [atorn, Zn(0H). |
4] nso, 9 ae
Mn0,(s) cue *
_ [avcom,c | [ance
| 7 | H,S0, NH, 11/ HCI
HO, K(Fe(CN),)
_ i K,Zn,(Fe(CN),1,(s)
| Mn*- Fe(OH),(s)
—__] HC,H,O,
elit K,[Fe(CN),)
| NaBiO,
Cu,{Fe(CN),1(8)
6 | H,S0,
KSCN
142
Laboratory Experiments for AP Chemistry
Experiment 19
Qualitative Analysis of Anions
Flow Chart
1 Agno,
AgCl(s) — AgBr(s)_—_Agl(s) 80,
| 1] NH,
TO
AgBris)Aalis) | aatsty) |
1| HNO,
ct
AgCl(s)
| Cr Br $0, CO> No, |
2| KMn0, |
| Mineral ot
| Laboratory Experiments for AP Chemistry 143
Experiment 19
Anions Flow Chart, continued
ct Br TF $0 CO% No-
3 HNO,
3 Ba(OH),
cr Br TF $0> co?
BaS0,(s) cr Br F NO;
cr Br F SO CO,
Lad Laboratory Experiments for AP Chemistry
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5814)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1092)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (844)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (540)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (348)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (822)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- RA5921 10918 ComparisonDocument50 pagesRA5921 10918 ComparisonAlfie1680% (40)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Assignment 1Document3 pagesAssignment 1Alfie16No ratings yet
- Vincent Gregory O. Mendoza 4BsphDocument2 pagesVincent Gregory O. Mendoza 4BsphAlfie16No ratings yet
- Paracetamol LabelDocument1 pageParacetamol LabelAlfie16No ratings yet
- Love For Sale: "If You Can't Receive It, Then Just Give It. Give Love, Tomasino!Document15 pagesLove For Sale: "If You Can't Receive It, Then Just Give It. Give Love, Tomasino!Alfie16No ratings yet
- IMRAD HandoutDocument1 pageIMRAD HandoutAlfie16100% (2)
- Pcol 2Document6 pagesPcol 2Alfie16No ratings yet
- Physcial Pharmacy Lec PrelimsDocument12 pagesPhyscial Pharmacy Lec PrelimsAlfie16No ratings yet
- AnxiolyticsDocument8 pagesAnxiolyticsAlfie16No ratings yet
- EthicsDocument3 pagesEthicsAlfie16No ratings yet
- Sampling of Raw Material Dispensing of Raw Material: On Pack" For Process Order"Document1 pageSampling of Raw Material Dispensing of Raw Material: On Pack" For Process Order"Alfie16No ratings yet
- Alfie Benedict P. Espedido 2BsphDocument1 pageAlfie Benedict P. Espedido 2BsphAlfie16No ratings yet
- Biochem LabDocument10 pagesBiochem LabAlfie16No ratings yet
- Lab 14Document13 pagesLab 14Alfie16No ratings yet
- Social OriginDocument5 pagesSocial OriginAlfie16No ratings yet
- Participant Treatment Sheet: This Sheet Is To Be Completed by The Prescribing DoctorDocument2 pagesParticipant Treatment Sheet: This Sheet Is To Be Completed by The Prescribing DoctorAlfie16No ratings yet
- CL, BR, I, SO, Co, NODocument3 pagesCL, BR, I, SO, Co, NOAlfie16No ratings yet
- Laboratory Experiments Qualitative AnalysisDocument62 pagesLaboratory Experiments Qualitative AnalysisAlfie16100% (1)
- Colors Memorization List: Flame Tests, Aqueous Ions, Compounds, Indicators Flame Test ColorsDocument1 pageColors Memorization List: Flame Tests, Aqueous Ions, Compounds, Indicators Flame Test ColorsAlfie16No ratings yet
- Org Chem ExDocument1 pageOrg Chem ExAlfie16No ratings yet
- Anion Schematic DiagramDocument1 pageAnion Schematic DiagramAlfie16No ratings yet