Professional Documents
Culture Documents
The Regenation of Catalyst
Uploaded by
Theoson HongOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
The Regenation of Catalyst
Uploaded by
Theoson HongCopyright:
Available Formats
CONCLUSION AND RECOMENDATION
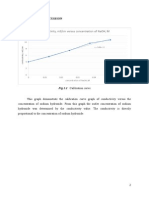
From the result of the experiment the conductivity of solution at the flowrate of
100mL/min in final CSTR is 3.60 while at the flowrate of 150 mL/min in the final
CSTR is 3.89. When compared to the calibration graph, the highest conversion at
flowrate of 100mL/min and 150mL/min are 0.97 and 0.94 respectively. In short, it
can be concluded that 100mL/min flow rate is more suitable than 150mL/min in the
saponification of ethyl acetate and sodium hydroxide in CSTR as higher conversion
and higher yield on saponification obtained in 100mL/min flowrate. Therefore, the
operating expenses can be minimised as the flow rate of solution is minimised but
with a higher yield on the desired product.
To obtain more precise experimental results in the future, several steps and
precautions should be taken to improve this experiment. Firstly, wash the conductivity
probe with distilled water every time before it used to measure the conductivity of
solution to ensure the accuracy of calibration graph. Secondly, make sure the tanks
and CSTR are clean and empty before the NaOH and Et(Ac) solution are charged into
the tanks. This can prevent the disturbance of other substances to the solutions. Next,
the condition such as temperature and stirrer speed of the CSTR should be aware by
the experimenter. Finally, the experimenters have to alert with the time taken. Wrong
timing can make the conductivity of the mixture change.
You might also like
- Saponification Reaction of Sodium Hydroxide An Ethyl Acetate in A Continuous-Stirred Tank Reactor (CSTR)Document21 pagesSaponification Reaction of Sodium Hydroxide An Ethyl Acetate in A Continuous-Stirred Tank Reactor (CSTR)drami94100% (13)
- D1795Document6 pagesD1795Ненад Кнежевић100% (2)
- Titrimetric Determination of CO2 in EthanolaminesDocument3 pagesTitrimetric Determination of CO2 in EthanolaminesDavinNo ratings yet
- 002.0 Surface Sampling TrainingDocument17 pages002.0 Surface Sampling TrainingNguyễnTrường100% (1)
- BSW Laboratory and Field TestDocument4 pagesBSW Laboratory and Field TestRonald Figo Torres Eche100% (1)
- Lab 5 Full ReportDocument9 pagesLab 5 Full Reporttirahanafi100% (1)
- Discussion Conclusion RecommendationDocument2 pagesDiscussion Conclusion RecommendationFaiz BasriNo ratings yet
- Rapid Determination of Benzalkonium Chloride in A Cosmetic: Key WordsDocument4 pagesRapid Determination of Benzalkonium Chloride in A Cosmetic: Key WordsYolby Milena Rodriguez ArizaNo ratings yet
- Determination of Oil and Grease in Water With A Mid-Infrared SpectrometerDocument4 pagesDetermination of Oil and Grease in Water With A Mid-Infrared SpectrometerVishal AroraNo ratings yet
- Nitrogen Bases in Hydrocarbons by Titration: UOP Method 269-10 ScopeDocument10 pagesNitrogen Bases in Hydrocarbons by Titration: UOP Method 269-10 ScopeEdwin CastilloNo ratings yet
- Informe Osmosis InversaDocument7 pagesInforme Osmosis InversaLina BeltranNo ratings yet
- 2.0 Results and Discussion: Fig 2.1 Calibration CurveDocument4 pages2.0 Results and Discussion: Fig 2.1 Calibration CurvemanorajNo ratings yet
- The New Equation of Steam Quality and The Evaluation of Nonradioactive Tracer Method in PWR Steam GeneratorsDocument13 pagesThe New Equation of Steam Quality and The Evaluation of Nonradioactive Tracer Method in PWR Steam GeneratorsJanice Carandang AldayNo ratings yet
- Conclusion and RecommendationDocument2 pagesConclusion and RecommendationHazirah AjamNo ratings yet
- Determination of Chloride in Water 4500DDocument3 pagesDetermination of Chloride in Water 4500Dpious_chemNo ratings yet
- Conclusion & Recommendation (Test Rig)Document1 pageConclusion & Recommendation (Test Rig)Noor FatihahNo ratings yet
- High-Temperature Continuous Bulk Copolymerization of Styrene and Acrylic Acid Determination of Monomer ConversionsDocument11 pagesHigh-Temperature Continuous Bulk Copolymerization of Styrene and Acrylic Acid Determination of Monomer ConversionsKumaranNo ratings yet
- Compounds Are Operated at This Range: 3.full Name of OOS & OOT ? Dercribe It With Details ? Why This Is Needed inDocument10 pagesCompounds Are Operated at This Range: 3.full Name of OOS & OOT ? Dercribe It With Details ? Why This Is Needed inRohit SharmaNo ratings yet
- Hydrogen Content of Gases by Gas ChromatographyDocument3 pagesHydrogen Content of Gases by Gas ChromatographyDavinNo ratings yet
- UV Spectrophotometric Determination of Theobromine and Caffeine in Cocoa BeansDocument4 pagesUV Spectrophotometric Determination of Theobromine and Caffeine in Cocoa BeansIwanOne'ajjNo ratings yet
- Met de Proveedor de Alfa AlfaDocument1 pageMet de Proveedor de Alfa AlfaErendira MarquezNo ratings yet
- A - Performance Tests Salt in Desalted Oil, Cameron Method C-010-CDocument6 pagesA - Performance Tests Salt in Desalted Oil, Cameron Method C-010-CSathish RajanNo ratings yet
- Chemical Engineering Laboratory Ii: /DT Term Is Zero SinceDocument9 pagesChemical Engineering Laboratory Ii: /DT Term Is Zero SinceKayathre Raveendran100% (1)
- A. Karim Et Al. / Catalysis Today 110 (2005) 86-91 87Document2 pagesA. Karim Et Al. / Catalysis Today 110 (2005) 86-91 87Widya AyuNo ratings yet
- Tutorial Absorption 2022Document27 pagesTutorial Absorption 2022Mars Studio0% (1)
- Trace Level Determmation of Heavy Metals in Drinking Water by Differential Pulse Anodic Stripping VoltammetryDocument7 pagesTrace Level Determmation of Heavy Metals in Drinking Water by Differential Pulse Anodic Stripping VoltammetryOmar ReynosoNo ratings yet
- Determination of Sugar As GlucoseDocument4 pagesDetermination of Sugar As GlucoseIpsita ChakravartyNo ratings yet
- A High Sensitive LC-MS/MS Method For Quantitation of Formoterol in Human PlasmaDocument1 pageA High Sensitive LC-MS/MS Method For Quantitation of Formoterol in Human PlasmaChristina WalkerNo ratings yet
- 4.0 Conclusion and Recommendation: M/MinDocument1 page4.0 Conclusion and Recommendation: M/MinTajTajNo ratings yet
- UOP 603 Trace CO and CO2 in Hydrogen and Light Gases Hydrocarbon by GCDocument6 pagesUOP 603 Trace CO and CO2 in Hydrogen and Light Gases Hydrocarbon by GCMorteza SepehranNo ratings yet
- CKB 20104 Reaction Engineering UniKL MICET Experiment 4: Reactor Test Rig Full Lab ReportDocument14 pagesCKB 20104 Reaction Engineering UniKL MICET Experiment 4: Reactor Test Rig Full Lab ReportSiti Hajar Mohamed100% (10)
- Two Issues Need To Be Prepared and Considered Before OperationDocument1 pageTwo Issues Need To Be Prepared and Considered Before Operationju_kaczaniukNo ratings yet
- Sample Lab ReportljDocument10 pagesSample Lab ReportljDavid DavisNo ratings yet
- Conclusion ReactorDocument1 pageConclusion ReactorzackwanNo ratings yet
- Bài Tập Phân Tích Công CụDocument38 pagesBài Tập Phân Tích Công Cụ12a50% (1)
- Means and Methods For Testing Adjustable Hydrostatic PumpsDocument7 pagesMeans and Methods For Testing Adjustable Hydrostatic Pumpsihp4romaniaNo ratings yet
- Exp 3 Osborne Reynold ApparatusDocument10 pagesExp 3 Osborne Reynold ApparatusnileshNo ratings yet
- Che314 Exp 5 ShonjaDocument8 pagesChe314 Exp 5 ShonjaSeele TlhagaNo ratings yet
- Sample Calculation For Efflux TimeDocument8 pagesSample Calculation For Efflux TimeLi Xue100% (1)
- 5990 3285en PDFDocument16 pages5990 3285en PDFLutfi CiludNo ratings yet
- Accidental Releases Analysis For Toxic Aqueous SolutionsDocument10 pagesAccidental Releases Analysis For Toxic Aqueous Solutionsvzimak2355No ratings yet
- HPLC 137.1Document9 pagesHPLC 137.1Rehana LebbeNo ratings yet
- Procedures: Safety Googles, Gloves and Lab Coat Were Worn When Handling These Chemicals)Document4 pagesProcedures: Safety Googles, Gloves and Lab Coat Were Worn When Handling These Chemicals)JxinLeeNo ratings yet
- USP Total Organic Carbon System Suitability Test For Aurora 1030W TOC Analyzer 2231Document8 pagesUSP Total Organic Carbon System Suitability Test For Aurora 1030W TOC Analyzer 2231Thu HàNo ratings yet
- DEMCON Method (For Evaluating Demulsifier Performance)Document17 pagesDEMCON Method (For Evaluating Demulsifier Performance)odracir091865No ratings yet
- Section 1 Gross Alpha and Gross Beta Radioactivity in Drinking Water METHOD 900.0 1.0 Scope and Application 1.1Document9 pagesSection 1 Gross Alpha and Gross Beta Radioactivity in Drinking Water METHOD 900.0 1.0 Scope and Application 1.1Pataki SandorNo ratings yet
- Heavy Oil Transportation As A SlurryDocument10 pagesHeavy Oil Transportation As A SlurryHomam MohammadNo ratings yet
- Cau 6 Chuong 2Document2 pagesCau 6 Chuong 2Sang PhanNo ratings yet
- ASTM Method D1945-96: Analysis of Natural GasDocument1 pageASTM Method D1945-96: Analysis of Natural GasJosep JaamNo ratings yet
- Normapur), With Various Different Concentrations.: 3.2. Pre-TreatmentDocument8 pagesNormapur), With Various Different Concentrations.: 3.2. Pre-TreatmentNidia CaetanoNo ratings yet
- Thermos OlDocument7 pagesThermos OlSridimalJayawardenaNo ratings yet
- Chapter 4. Problem SM.7 - Ethylbenzene-Styrene Column PDFDocument11 pagesChapter 4. Problem SM.7 - Ethylbenzene-Styrene Column PDFMary ScottNo ratings yet
- Boiler Water Test Procedures CompleteDocument25 pagesBoiler Water Test Procedures Completedso0% (4)
- Experiment 6Document5 pagesExperiment 6Annam AsgharNo ratings yet
- PNEUMATICS AND AIR CIRCUITS UNDERSTANDING THE CASCADE VALVE AND PLC UNDERSTANDINGFrom EverandPNEUMATICS AND AIR CIRCUITS UNDERSTANDING THE CASCADE VALVE AND PLC UNDERSTANDINGNo ratings yet
- Enhanced Oil Recovery: Resonance Macro- and Micro-Mechanics of Petroleum ReservoirsFrom EverandEnhanced Oil Recovery: Resonance Macro- and Micro-Mechanics of Petroleum ReservoirsRating: 5 out of 5 stars5/5 (1)