Professional Documents
Culture Documents
Osmosis PPT 2
Osmosis PPT 2
Uploaded by
api-3098934090 ratings0% found this document useful (0 votes)
151 views4 pagesOsmosis is the diffusion of water molecules through a partially permeable membrane from an area of higher water concentration to lower water concentration. It can be demonstrated using visking tubing filled with a solution placed in a beaker of pure water. The water molecules will diffuse through the membrane into the visking tubing. A dilute solution has a high concentration of water molecules while a concentrated solution has a low concentration of water molecules, so water will diffuse from the dilute to the concentrated solution through the membrane.
Original Description:
Original Title
osmosis ppt 2
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentOsmosis is the diffusion of water molecules through a partially permeable membrane from an area of higher water concentration to lower water concentration. It can be demonstrated using visking tubing filled with a solution placed in a beaker of pure water. The water molecules will diffuse through the membrane into the visking tubing. A dilute solution has a high concentration of water molecules while a concentrated solution has a low concentration of water molecules, so water will diffuse from the dilute to the concentrated solution through the membrane.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
151 views4 pagesOsmosis PPT 2
Osmosis PPT 2
Uploaded by
api-309893409Osmosis is the diffusion of water molecules through a partially permeable membrane from an area of higher water concentration to lower water concentration. It can be demonstrated using visking tubing filled with a solution placed in a beaker of pure water. The water molecules will diffuse through the membrane into the visking tubing. A dilute solution has a high concentration of water molecules while a concentrated solution has a low concentration of water molecules, so water will diffuse from the dilute to the concentrated solution through the membrane.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
You are on page 1of 4
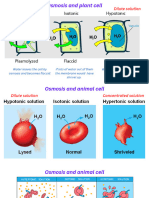
Osmosis
Topic B1 and B2
LO To define and describe the importance of osmosis
Osmosis is the diffusion of water molecules from a region of their
higher concentration (dilute solution) to a region of their lower
concentration (concentrated solution), through a partially permeable
membrane >
What is osmosis?
Osmosis is the diffusion of water molecules from a low
concentration solution to a high concentration solution
across a partially-permeable membrane.
A partially-permeable membrane has holes in it that permit
water molecules through, but are too small to allow larger
molecules through. Osmosis can be demonstrated using
visking tubing filled with a solution and placed in a beaker
of pure water.
partiallypermeable
membrane
(visking tubing)
water
glucose
Dilute vs. concentrated
During osmosis, water molecules diffuse from pure water or
dilute solution to more concentrated solutions.
Dilute solutions have a high concentration of
water molecules.
Concentrated solutions have a low concentration
of water molecules.
pure water
dilute solution
concentrated
solution
You might also like
- 10 3 Tropic Response ppt2Document37 pages10 3 Tropic Response ppt2api-309893409No ratings yet
- Dna Structure gp13Document3 pagesDna Structure gp13api-309893409No ratings yet
- Chapter 3 - Diffusion & OsmosisDocument26 pagesChapter 3 - Diffusion & Osmosisapi-3728508100% (6)
- Leaf Structure WorksheetDocument1 pageLeaf Structure Worksheetapi-309893409100% (1)
- Aerobic RespirationDocument35 pagesAerobic Respirationapi-309893409100% (3)
- Leaves and PhotosynthesisDocument18 pagesLeaves and Photosynthesisapi-309893409100% (1)
- Reverse Osmosis Treatment of Drinking WaterFrom EverandReverse Osmosis Treatment of Drinking WaterRating: 3.5 out of 5 stars3.5/5 (4)
- Chem123 Lab Notes PrelimDocument1 pageChem123 Lab Notes PrelimKristine PangahinNo ratings yet
- Unit-I-IMTH-III (Osmosis, Types of Solution & Plasmolysis) PDFDocument47 pagesUnit-I-IMTH-III (Osmosis, Types of Solution & Plasmolysis) PDFanuksha aroraNo ratings yet
- Osmosis and Semipermeable MembranesDocument4 pagesOsmosis and Semipermeable MembranesAarhan KhanNo ratings yet
- Tonicity: Isotonic SolutionDocument5 pagesTonicity: Isotonic SolutionGembelle MayorgaNo ratings yet
- Osmosis ReportDocument14 pagesOsmosis ReportKhai Zyann OcayNo ratings yet
- Theory: 1 Plant Water RelationsDocument11 pagesTheory: 1 Plant Water RelationsalekhyaNo ratings yet
- B3.2a OsmosisDocument14 pagesB3.2a Osmosis叶冰裳No ratings yet
- Diffusion: The More Particles There Are in A Certain Volume, The More Concentrated Those Particles AreDocument6 pagesDiffusion: The More Particles There Are in A Certain Volume, The More Concentrated Those Particles AreGia Espinosa OcbeñaNo ratings yet
- Bio Diffusion OsmosisDocument9 pagesBio Diffusion OsmosisRodge BuereNo ratings yet
- Osmosis, Diffusion & The Cell Membrane DiffusionDocument3 pagesOsmosis, Diffusion & The Cell Membrane DiffusionNo OneNo ratings yet
- Reverse OsmosisDocument3 pagesReverse OsmosisJOSEPH HERBERT MABELNo ratings yet
- Passive TransportDocument11 pagesPassive Transportdanielle stephanieNo ratings yet
- Diffusion Vs OsmosisDocument3 pagesDiffusion Vs OsmosisK TanNo ratings yet
- 3 Diffusion & OsmosisDocument27 pages3 Diffusion & OsmosisnasserkhanNo ratings yet
- Cell Transport: by Felipe Hoffmann, Juan Felipe Canal, Juan Sebastian Lopez, Nicolas Castrillon and Simon KnudsenDocument6 pagesCell Transport: by Felipe Hoffmann, Juan Felipe Canal, Juan Sebastian Lopez, Nicolas Castrillon and Simon KnudsenSimon KnudsenNo ratings yet
- Hypotonic Because It Has Less of The Solute Molecules, But More of TheDocument1 pageHypotonic Because It Has Less of The Solute Molecules, But More of TheCaitlyn9777No ratings yet
- Osmosis Presentation@Document20 pagesOsmosis Presentation@Dima ArafatNo ratings yet
- Lec.4 Diffusion and OsmosisDocument15 pagesLec.4 Diffusion and OsmosisSahar HassanNajadNo ratings yet
- Reverse Osmosis BasicsDocument3 pagesReverse Osmosis BasicsJohnNo ratings yet
- B1.7 OsmosisDocument3 pagesB1.7 OsmosisYevonNo ratings yet
- Movement of Molecules Notes (Year 10 Biology)Document16 pagesMovement of Molecules Notes (Year 10 Biology)Janki patelNo ratings yet
- REVISION NOTES - B2.2MovementInAndOutOfTheCellDocument4 pagesREVISION NOTES - B2.2MovementInAndOutOfTheCellDavé TranNo ratings yet
- OsmosisDocument19 pagesOsmosisdamyangdcsNo ratings yet
- Roll No. 1624407: GURVINDER K. Sandhu S.Y. BSC Tranport Across The MembraneDocument20 pagesRoll No. 1624407: GURVINDER K. Sandhu S.Y. BSC Tranport Across The MembraneRupali GuravNo ratings yet
- Review Tests Subs - Mapeh, Biotech, Math Quarter 2Document4 pagesReview Tests Subs - Mapeh, Biotech, Math Quarter 2zabala.vanessa.sixdaffodilNo ratings yet
- Osmosis: Diffusion Is The Process Where Molecules of Two or More Substances Move AboutDocument3 pagesOsmosis: Diffusion Is The Process Where Molecules of Two or More Substances Move AboutizzahNo ratings yet
- Osmosis QuestionsDocument13 pagesOsmosis QuestionsByambazaya ENo ratings yet
- Diffusion and OsmosisDocument3 pagesDiffusion and OsmosisRenee Dwi Permata MessakaraengNo ratings yet
- Passive TransportDocument3 pagesPassive Transportirnumambreen15No ratings yet
- Difussion-And-Osmosis-Reviewer - 31-40 (Solutions)Document6 pagesDifussion-And-Osmosis-Reviewer - 31-40 (Solutions)Vienna GilmoreNo ratings yet
- Plant Physiolog-WPS OfficeDocument45 pagesPlant Physiolog-WPS OfficeShubhendu ChattopadhyayNo ratings yet
- Diffusion and Osmosis - IGCSEDocument37 pagesDiffusion and Osmosis - IGCSEJohnnie ZhangNo ratings yet
- Biology 5090-Diffusionosmosis-And-Active-Transport - pptx-1Document36 pagesBiology 5090-Diffusionosmosis-And-Active-Transport - pptx-1Saba AnjumNo ratings yet
- Effects of Immersing Plant Tissues With Varying WaterDocument7 pagesEffects of Immersing Plant Tissues With Varying WaterMarcus GohNo ratings yet
- Transport Across MembraneDocument5 pagesTransport Across MembraneRena Emmanuelle PalenciaNo ratings yet
- Cells: Diffusion and OsmosisDocument22 pagesCells: Diffusion and OsmosisjmgardnerNo ratings yet
- 06-Movement of Materials-1Document11 pages06-Movement of Materials-1Maku MichaelNo ratings yet
- Diffusion & OsmosisDocument19 pagesDiffusion & Osmosishugo roblesNo ratings yet
- Movement in and Out of CellsDocument18 pagesMovement in and Out of CellsEmy AnkrahNo ratings yet
- Diffusion and Osmosis WorksheetDocument3 pagesDiffusion and Osmosis Worksheetohmyveenus617No ratings yet
- Biochem Laboratory Activity 2Document4 pagesBiochem Laboratory Activity 2Kristine PangahinNo ratings yet
- Bio3 TransiDocument47 pagesBio3 TransichemasuetNo ratings yet
- PLANT-PHYSIOLOGYDocument33 pagesPLANT-PHYSIOLOGYselesmabNo ratings yet
- 1 Osmosis DialysisDocument5 pages1 Osmosis DialysisRio SurNo ratings yet
- OsmosisDocument2 pagesOsmosisUmasalam OdawaNo ratings yet
- Osmosis and Diffusion - 2nd YearDocument13 pagesOsmosis and Diffusion - 2nd YearFrancisco Samarco100% (1)
- Movement in and Out of CellsDocument10 pagesMovement in and Out of CellsarhampracticeNo ratings yet
- HURDCO International School: Subject-Biology Chapter 3: Diffusion, Osmosis and Surface Area: Volume RatioDocument26 pagesHURDCO International School: Subject-Biology Chapter 3: Diffusion, Osmosis and Surface Area: Volume RatioMahin IslamNo ratings yet
- OsmosisDocument3 pagesOsmosisgrace.a.sun18No ratings yet
- Movement in and Out of Cells NotesDocument9 pagesMovement in and Out of Cells Notesmanarehab35No ratings yet
- Reverse Osmosis Basics - Toray Reverse Osmosis Basics - Knowledge Base - Toray Membrane - TorayDocument2 pagesReverse Osmosis Basics - Toray Reverse Osmosis Basics - Knowledge Base - Toray Membrane - ToraySantosh Kumar SinghNo ratings yet
- Cellular Transport 0910Document23 pagesCellular Transport 0910Ronald Chester LorenzanaNo ratings yet
- Worksheet KeyDocument2 pagesWorksheet KeyjmgardnerNo ratings yet
- Osmosis: From Wikipedia, The Free EncyclopediaDocument6 pagesOsmosis: From Wikipedia, The Free Encyclopediaallanlopez_2009No ratings yet
- Chem ReportDocument15 pagesChem ReportMaria Fatima SagaoinitNo ratings yet
- Osmosis: 1 MechanismDocument6 pagesOsmosis: 1 MechanismsnowflomanNo ratings yet
- MOvement in and Out of Cells - Osmosis & Active TransportDocument4 pagesMOvement in and Out of Cells - Osmosis & Active TransportTshepo O MotsemmeNo ratings yet
- Alimentary Canal ws1Document5 pagesAlimentary Canal ws1api-309893409No ratings yet
- CholeraDocument10 pagesCholeraapi-309893409No ratings yet
- Respiration InvestigationDocument3 pagesRespiration Investigationapi-309893409No ratings yet
- Alimentary CanalDocument29 pagesAlimentary Canalapi-309893409100% (1)
- LabourDocument8 pagesLabourapi-309893409No ratings yet
- Reproduction WsDocument6 pagesReproduction Wsapi-309893409100% (1)
- Circus WorksheetsDocument16 pagesCircus Worksheetsapi-309893409No ratings yet
- Respiration HomeworkDocument8 pagesRespiration Homeworkapi-309893409No ratings yet
- Respirometer PPQDocument3 pagesRespirometer PPQapi-309893409No ratings yet
- Fertilisation and PregnancyDocument33 pagesFertilisation and Pregnancyapi-309893409No ratings yet
- Plant Nutrition RevisionDocument1 pagePlant Nutrition Revisionapi-3098934090% (1)
- A Sexual and Sexual Card SortDocument2 pagesA Sexual and Sexual Card Sortapi-309893409100% (1)
- PhotosynthesisDocument13 pagesPhotosynthesisapi-309893409No ratings yet
- Hydrogen Carbonate Practical Set UpDocument1 pageHydrogen Carbonate Practical Set Upapi-309893409No ratings yet
- Information Sheet 1Document1 pageInformation Sheet 1api-309893409No ratings yet
- Past Exam QuestionDocument1 pagePast Exam Questionapi-309893409No ratings yet
- SummaryDocument1 pageSummaryapi-309893409No ratings yet
- 1 Innate ImmunityDocument12 pages1 Innate Immunityapi-309893409No ratings yet
- EnzymesDocument17 pagesEnzymesapi-309893409No ratings yet
- Active ImmunityDocument17 pagesActive Immunityapi-309893409No ratings yet
- H8a - A Bugs LifeDocument2 pagesH8a - A Bugs Lifeapi-309893409No ratings yet
- Worksheet - Tropic Response QsDocument3 pagesWorksheet - Tropic Response Qsapi-309893409No ratings yet