Professional Documents
Culture Documents
Subscripts KEY: FIG. 25-1 Nomenclature
Uploaded by
Gerardo Mediavilla0 ratings0% found this document useful (0 votes)
12 views2 pagestube
Original Title
Section25.xlsx
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documenttube
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
12 views2 pagesSubscripts KEY: FIG. 25-1 Nomenclature
Uploaded by
Gerardo Mediavillatube
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
FIG.
25-1
Nomenclature
Ki
L
P*
R
=
=
= equilibrium ratio, yi/xi
= ratio of moles of liquid to moles of total
mixture
= mole fraction in the total mixture or system
= acentric factor
= absolute pressure, psia
xi
Pk
= convergence pressure, psia
yi
Subscripts
KEY
= Example calculation from the book
= Application worksheet for user to fill out
= Numbers that must be filled in according to the user's data and specific situation (also includes numbers
vapor pressure, psia
universal gas constant,
(psia cu ft)/(lbmole R)
temperature, R or F
ratio of moles of vapor to moles of total
pressure
mole fraction of component I in the liquid
phase
mole fraction of component I in the vapor
phase
component
ific situation (also includes numbers from graphs and charts)
You might also like
- Working Guide to Vapor-Liquid Phase Equilibria CalculationsFrom EverandWorking Guide to Vapor-Liquid Phase Equilibria CalculationsRating: 5 out of 5 stars5/5 (1)
- Abstract Vle ExperimentDocument5 pagesAbstract Vle ExperimentjuaxxoNo ratings yet
- Vap EquilibriumDocument14 pagesVap EquilibriumPRAVINNo ratings yet
- Surface Tension Prediction For Liquid Mixtures: Aiche Journal, Volume 44, No. 10, Pp. 2324-2332, October 1998Document23 pagesSurface Tension Prediction For Liquid Mixtures: Aiche Journal, Volume 44, No. 10, Pp. 2324-2332, October 1998Dnyaneshwar GavandeNo ratings yet
- Vle 4Document15 pagesVle 4PRAVINNo ratings yet
- Binary Vapor Liquid EquilibriumDocument7 pagesBinary Vapor Liquid EquilibriumBiain A SecasNo ratings yet
- PRO-II Thermodynamic Model SelectionDocument79 pagesPRO-II Thermodynamic Model Selectionchemsac2100% (1)
- Multicomponent and Multiphase SystemsDocument15 pagesMulticomponent and Multiphase SystemsZain AliNo ratings yet
- Ceic3001 NotesDocument3 pagesCeic3001 NotesZooy2012No ratings yet
- Thermodynamic Properties Chart CorrelationsDocument44 pagesThermodynamic Properties Chart CorrelationsDavid RomeroNo ratings yet
- Vapor Liquid Equilibria: A Review: Maya B. Mane and S. N. ShindeDocument15 pagesVapor Liquid Equilibria: A Review: Maya B. Mane and S. N. ShindeDesi Riana SaputriNo ratings yet
- Vapour Liquid EquilibriumDocument11 pagesVapour Liquid EquilibriumPRAVINNo ratings yet
- Week 2 - Vle Part 1Document35 pagesWeek 2 - Vle Part 1dhanieemaNo ratings yet
- M24 - Thermodynamic PropertiesDocument38 pagesM24 - Thermodynamic Propertieshoghost123No ratings yet
- Tablas y Figura 24Document42 pagesTablas y Figura 24GiovanyBrachoNo ratings yet
- Vapour Liquid EquilibriumDocument15 pagesVapour Liquid EquilibriumPRAVINNo ratings yet
- TextDocument4 pagesTextMd Masum BillahNo ratings yet
- Separation Process Engineering CHEN 312: Ys18@aub - Edu.lbDocument28 pagesSeparation Process Engineering CHEN 312: Ys18@aub - Edu.lbsoe0303No ratings yet
- GPSA Propiedades Termodinamicas 24 PDFDocument42 pagesGPSA Propiedades Termodinamicas 24 PDFDavid Cortez PeraltaNo ratings yet
- Termodinamica de Hidrocarburos: Generalized Phase EquilibriaDocument109 pagesTermodinamica de Hidrocarburos: Generalized Phase Equilibria13670319No ratings yet
- Simple Functions For Fast Calculations of Selected Thermodynamic Properties of The Ammonia-Water SystemDocument7 pagesSimple Functions For Fast Calculations of Selected Thermodynamic Properties of The Ammonia-Water SystemEngineer1987No ratings yet
- Week 2 - Vle Part 1Document35 pagesWeek 2 - Vle Part 1Syed Hassan Syed Hashim100% (1)
- AlgoDocument46 pagesAlgoJoseCastilhoNo ratings yet
- CET-II Chapter 1 Vapour-Liquid Equilibrium - Part 1Document29 pagesCET-II Chapter 1 Vapour-Liquid Equilibrium - Part 1Dhruv RanaNo ratings yet
- 01 Brambilla CH 01Document28 pages01 Brambilla CH 01kumar_chemicalNo ratings yet
- VLE: Vapor Liquid EquilibriumDocument39 pagesVLE: Vapor Liquid EquilibriumTouhid IslamNo ratings yet
- 0378 38122987010 7Document15 pages0378 38122987010 7Tiên PhạmNo ratings yet
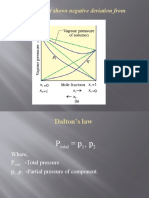
- (B) A Solution That Shows Negative Deviation From Raoult's LawDocument9 pages(B) A Solution That Shows Negative Deviation From Raoult's LawPRAVINNo ratings yet
- M24 Phisical Properties GPSADocument42 pagesM24 Phisical Properties GPSAPawan ChaturvediNo ratings yet
- Dalton's Law: Where, P - Total Pressure P, P - Partial Pressure of ComponentDocument12 pagesDalton's Law: Where, P - Total Pressure P, P - Partial Pressure of ComponentPRAVINNo ratings yet
- Chemical Engineering Thermodynamics IIIIIIIIDocument14 pagesChemical Engineering Thermodynamics IIIIIIIIDarnell HendersonNo ratings yet
- Experiments in General Chemistry Goldwhite Tikkanen (Dragged) 2 1Document1 pageExperiments in General Chemistry Goldwhite Tikkanen (Dragged) 2 1Anonymous zHmefGH30YNo ratings yet
- Review of Phase Equilibria - NEWDocument19 pagesReview of Phase Equilibria - NEWkarmawii taqatqaNo ratings yet
- Vapiquid IbriumDocument15 pagesVapiquid IbriumPRAVINNo ratings yet
- Chemical Engineering Thermodynamics Project-I: TopicDocument11 pagesChemical Engineering Thermodynamics Project-I: TopicRohit GuptaNo ratings yet
- Calculation of Vapor-Liquid-Liquid Equilibria For The Fischer-Tropsch Reactor Effluents Using Modified Peng-Robinson Equation of StateDocument31 pagesCalculation of Vapor-Liquid-Liquid Equilibria For The Fischer-Tropsch Reactor Effluents Using Modified Peng-Robinson Equation of StatekenymorenoNo ratings yet
- Chemical Engineering Thermodynamics -II(2150503) Fugacity & Fugacity CoefficientDocument21 pagesChemical Engineering Thermodynamics -II(2150503) Fugacity & Fugacity CoefficientLaiba JanjuaNo ratings yet
- Fe Chemical EngineeringDocument5 pagesFe Chemical EngineeringJudith LugoNo ratings yet
- CL-201 Chapter 6 Multiphase Systems (Compatibility Mode) PDFDocument42 pagesCL-201 Chapter 6 Multiphase Systems (Compatibility Mode) PDFSuman MandalNo ratings yet
- Raoult's Law, Fugacity, Activity Coefficients, and Methods for Calculating Activity CoefficientsDocument14 pagesRaoult's Law, Fugacity, Activity Coefficients, and Methods for Calculating Activity CoefficientsPRAVINNo ratings yet
- Calculating Fugacity of Pure Liquid n-PentaneDocument8 pagesCalculating Fugacity of Pure Liquid n-PentanescienziatoNo ratings yet
- 1-Vle Part 1Document30 pages1-Vle Part 1Arfa Zulkifli01No ratings yet
- Distillation TheoryDocument40 pagesDistillation TheoryIrvin HernandezNo ratings yet
- Vapor Liquid EquilibriumDocument39 pagesVapor Liquid EquilibriumJakeWilliam100% (1)
- Humidity in AirDocument6 pagesHumidity in AirJeffNo ratings yet
- Diagram SketchingDocument3 pagesDiagram SketchingQuennie Marie AñanaNo ratings yet
- Chapter 6 - Multiphase Systems: CBE2124, LevickyDocument27 pagesChapter 6 - Multiphase Systems: CBE2124, LevickyRimmonNo ratings yet
- Vapour Liquid EquilibriumDocument32 pagesVapour Liquid EquilibriumHaseen Kaur0% (1)
- Solution Thermodynamics Theory-Ch 11Document50 pagesSolution Thermodynamics Theory-Ch 11Donni Azhar100% (2)
- A Self-Consistent GE MR For CEoS Derivation and Fugacity CoefficientsDocument4 pagesA Self-Consistent GE MR For CEoS Derivation and Fugacity Coefficientsmurdanetap957No ratings yet
- Lecture-8,9,10 VLE DiagramsDocument64 pagesLecture-8,9,10 VLE DiagramsShiavm PatelNo ratings yet
- ChE 152 Lecture 2a Vapor-Liquid EquilibriaDocument33 pagesChE 152 Lecture 2a Vapor-Liquid EquilibriaEmrico Luiz PerezNo ratings yet
- Enthalpy Calculation Two Phase Binary MixtureDocument2 pagesEnthalpy Calculation Two Phase Binary MixtureMaurice PolitisNo ratings yet
- Psychrometric ChartDocument18 pagesPsychrometric ChartFafaNo ratings yet
- ChE 3G4 Spreadsheet Flash CalculationsDocument9 pagesChE 3G4 Spreadsheet Flash CalculationsRafael Reyes0% (1)
- Thermodynamics Selection in Chemcad1Document39 pagesThermodynamics Selection in Chemcad1Yashpal MalikNo ratings yet
- Pure Component VLE in Terms of Fugacity: LiquidsDocument8 pagesPure Component VLE in Terms of Fugacity: Liquidsahad_shiraziNo ratings yet
- Thermodynamic Models for Chemical Engineering: Design, Develop, Analyse and OptimizeFrom EverandThermodynamic Models for Chemical Engineering: Design, Develop, Analyse and OptimizeNo ratings yet
- Recommended Reference Materials for Realization of Physicochemical Properties: Pressure–Volume–Temperature RelationshipsFrom EverandRecommended Reference Materials for Realization of Physicochemical Properties: Pressure–Volume–Temperature RelationshipsE. F. G. HeringtonNo ratings yet
- NDT CP Asnt PDFDocument5 pagesNDT CP Asnt PDFG Prabhakar RajuNo ratings yet
- Extracted Pages From... ASME VIII - DIV.2 (2019) - 3Document1 pageExtracted Pages From... ASME VIII - DIV.2 (2019) - 3Gerardo MediavillaNo ratings yet
- Dmas d.amsd.as mdasDocument1 pageDmas d.amsd.as mdasGerardo MediavillaNo ratings yet
- NDT CP Asnt PDFDocument5 pagesNDT CP Asnt PDFG Prabhakar RajuNo ratings yet
- ASNT L3 ApplicationDocument13 pagesASNT L3 ApplicationAbdur RahimNo ratings yet
- Astm E1220Document6 pagesAstm E1220Gerardo Mediavilla100% (1)
- Astm e 587 - 2015Document9 pagesAstm e 587 - 2015Gerardo MediavillaNo ratings yet
- Astm E2700Document9 pagesAstm E2700Gerardo MediavillaNo ratings yet
- Leak Detection Methods: A Comparative Study of Technologies and Techniques Short VersionDocument24 pagesLeak Detection Methods: A Comparative Study of Technologies and Techniques Short VersionHappy202180% (5)
- Documento 127Document3 pagesDocumento 127Gerardo MediavillaNo ratings yet
- Astm e 797 - 2015Document7 pagesAstm e 797 - 2015Gerardo Mediavilla100% (3)
- E797e797m 1657Document7 pagesE797e797m 1657Gerardo MediavillaNo ratings yet
- Proc 02Document3 pagesProc 02Gerardo MediavillaNo ratings yet
- Documento 129Document5 pagesDocumento 129Gerardo MediavillaNo ratings yet
- Cu89 PDFDocument2 pagesCu89 PDFGerardo MediavillaNo ratings yet
- Documento 128Document4 pagesDocumento 128Gerardo MediavillaNo ratings yet
- Cu 89Document2 pagesCu 89Gerardo MediavillaNo ratings yet
- A135A135MDocument9 pagesA135A135Msamy7354No ratings yet
- TOOSSDocument1 pageTOOSSGerardo MediavillaNo ratings yet
- Steel Structure 001Document1 pageSteel Structure 001Gerardo MediavillaNo ratings yet
- WeldingDocument129 pagesWeldingAnilkumar Cm93% (15)