Professional Documents
Culture Documents
I. Einstein'S Theory of Brownian Motion
Uploaded by
Cristina Sofia PinneriOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
I. Einstein'S Theory of Brownian Motion
Uploaded by
Cristina Sofia PinneriCopyright:
Available Formats
1
I.
EINSTEINS THEORY OF BROWNIAN MOTION
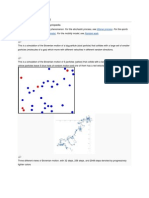
Brownian motion, first observed by the botanist *** Brown in 18***, consists in an erratic motion of micromeric
particles in suspension in a liquid and can be seen with a microscope. Possible explanations in terms of convective
motion of the solvent failed to give account of the observation. The theory of Einstein in 1905 esablishes that the
motion is a macroscopic manifestation of 1) the granular (atomic) nature of matter, 2) the molecular chaos. At
the time of Einstein, the atomic hypothesis originated in chemistry was still much debated, despite the success of
statistical mechanics in explaining thermodynamics in mechanicistic terms. Molecular chaos was a concept introduced
by Bolzmann postulating maximal disorder and essentially independence in the motion of molecules in diluted gases,
to derive an equation -the Bolzmann equation- describing the relaxation to equilibrium of these gases.
In order to explain Brownian motion, Einstein first observation is that according to the atomic hypothesis a
suspension should differ from a solution solely for the size of the particles in solution. Accordingly, a suspention
separated by a semi-permeable membrane from a fluid should exhert an osmotic pressure on the fluid.
In the diluted limit in which it is possible to neglect the interaction between the particles of the solute it is easy to
derive, from statistical mechanics, the law of perfect gases. If V is the volume occupied by the macroscopic particles,
and there are N of the probability at equilibrium of findinng the N particles in the phase space element d3N xd3 N p
is proportional to
!
N
X
2
p(x, p) exp
pi /(2KB T )
(1)
i=1
the probability of the position of each particle is uniform in space, while the momena follow the Maxwell disribution;
T is the temperature, KB the Boltzmann constant. According to thermodynamics, the osmotic pressure can be
computed as p = F /V . According to statistical physics, the free-energy is related to the partition function Z
by F = KB T log Z with
Z =
(2)
You might also like
- Einstein, Albert - A New Determination of Molecular Dimensions - 1905Document16 pagesEinstein, Albert - A New Determination of Molecular Dimensions - 1905nicopanzNo ratings yet
- The Particle Problem in The General Theory of RelativityDocument5 pagesThe Particle Problem in The General Theory of RelativitymargonitoNo ratings yet
- Random Vibration - A Brief HistoryDocument9 pagesRandom Vibration - A Brief HistorykavskarNo ratings yet
- Quasi Particles Kaganov LifshitsDocument100 pagesQuasi Particles Kaganov Lifshitsamlferreira100% (1)
- Brownian MotionDocument76 pagesBrownian MotionPraveen KumarNo ratings yet
- Brownian Dynamics 2014Document112 pagesBrownian Dynamics 2014Ali MansourNo ratings yet
- The Einstein-Myth and The Crisis in Modern PhysicsDocument17 pagesThe Einstein-Myth and The Crisis in Modern PhysicsLuis Gerardo Escandon AlcazarNo ratings yet
- Investigations On The Theory of The Brownian MovementDocument11 pagesInvestigations On The Theory of The Brownian MovementRafael Poerschke100% (1)
- [Cambridge Mathematical Library] Sydney Chapman, T. G. Cowling, C. Cercignani - The mathematical theory of non-uniform gases_ an account of the kinetic theory of viscosity, thermal conduction, and diffusion in gases (1995, Cambrid.pdfDocument448 pages[Cambridge Mathematical Library] Sydney Chapman, T. G. Cowling, C. Cercignani - The mathematical theory of non-uniform gases_ an account of the kinetic theory of viscosity, thermal conduction, and diffusion in gases (1995, Cambrid.pdfLorenzo CampoliNo ratings yet
- Heisenberg's Uncertainty Principle and Wave-Particle Dualism.Document5 pagesHeisenberg's Uncertainty Principle and Wave-Particle Dualism.Bezverkhniy VolodymyrNo ratings yet
- Fokker Planck EquationDocument13 pagesFokker Planck EquationRufuNo ratings yet
- Derivation of The Schrodinger Equation From Newtonian MechanicsDocument7 pagesDerivation of The Schrodinger Equation From Newtonian MechanicsCyrille TijsselingNo ratings yet
- Lecture 6: Entropy: Tot TotDocument21 pagesLecture 6: Entropy: Tot TotabcdefNo ratings yet
- Brownian Motion of Stars, Dust, and Invisible Matter: Edmund BertschingerDocument9 pagesBrownian Motion of Stars, Dust, and Invisible Matter: Edmund BertschingerRalfRalfNo ratings yet
- Entropy: Gibbs' Paradox and The Definition of EntropyDocument4 pagesEntropy: Gibbs' Paradox and The Definition of Entropynoro70No ratings yet
- Entropy of An Isolated System: Ludwig Boltzmann and EntropyDocument3 pagesEntropy of An Isolated System: Ludwig Boltzmann and EntropyPawan KumarNo ratings yet
- Kinetic TheoryDocument20 pagesKinetic Theorykishorkumarn8212No ratings yet
- A Review On Natural Reality With Physical EquationsDocument7 pagesA Review On Natural Reality With Physical EquationsMia AmaliaNo ratings yet
- Kinetic TheoryDocument7 pagesKinetic TheoryngvanduysnNo ratings yet
- Chanwoo - CDR Pdfs Indexed 1 1Document9 pagesChanwoo - CDR Pdfs Indexed 1 1fsgfdgsgfgfsNo ratings yet
- Brownian Motion: From Wikipedia, The Free EncyclopediaDocument21 pagesBrownian Motion: From Wikipedia, The Free EncyclopediarayroyNo ratings yet
- Richard Easther Et Al - Brane Gases in The Early Universe: Thermodynamics and CosmologyDocument35 pagesRichard Easther Et Al - Brane Gases in The Early Universe: Thermodynamics and CosmologyHuntsmithNo ratings yet
- Entropy: Gibbs' Paradox and The Definition of EntropyDocument4 pagesEntropy: Gibbs' Paradox and The Definition of Entropysantiago bermudezNo ratings yet
- Applied Mathematical Modelling: Ben Evans, Ken Morgan, Oubay HassanDocument20 pagesApplied Mathematical Modelling: Ben Evans, Ken Morgan, Oubay HassanMark HannaNo ratings yet
- Time and Space Scales in Brownian MotionDocument8 pagesTime and Space Scales in Brownian MotionFrank MunleyNo ratings yet
- Kinetic Theory of GasessDocument9 pagesKinetic Theory of GasessTchierry S PurhooaNo ratings yet
- Statistical Mechanics SolutionsDocument17 pagesStatistical Mechanics SolutionsZahra KhanNo ratings yet
- Kinetics of EvaporationDocument14 pagesKinetics of Evaporationfluffa23No ratings yet
- Physical: Review LettersDocument2 pagesPhysical: Review LettersEnrique PugaNo ratings yet
- PhysRevLett 97-CsernaiDocument4 pagesPhysRevLett 97-CsernaiNoah MacKayNo ratings yet
- History of HeatDocument31 pagesHistory of Heatkarlo marko sta. rosaNo ratings yet
- The Reinterpretation of Wave Mechanics: Louis de BroglieDocument11 pagesThe Reinterpretation of Wave Mechanics: Louis de BrogliecerconeNo ratings yet
- Gleb Finkelstein-Statistical MechanicsDocument42 pagesGleb Finkelstein-Statistical MechanicsH LNo ratings yet
- Kinetic Theory of Gases: A Brief Review: Michael Fowler 6/5/08Document13 pagesKinetic Theory of Gases: A Brief Review: Michael Fowler 6/5/08khalidanwar21No ratings yet
- Bose-Einstein CondensateDocument20 pagesBose-Einstein CondensateNirmal BhowmickNo ratings yet
- Susanne La Mura - The Bose-Einstein Condensate: How Information Technology Helps To Push On Physical AchievementsDocument3 pagesSusanne La Mura - The Bose-Einstein Condensate: How Information Technology Helps To Push On Physical AchievementsLomewcxNo ratings yet
- Einstein Dissertation English PDFDocument15 pagesEinstein Dissertation English PDFlimberg_maytaNo ratings yet
- Brownian Dynamics 2014Document112 pagesBrownian Dynamics 2014Ali MansourNo ratings yet
- Kubo Fluctuation-Dissipation TheoremDocument30 pagesKubo Fluctuation-Dissipation Theoremmbiswas51No ratings yet
- 9205 Chap01Document18 pages9205 Chap01Shridhar MathadNo ratings yet
- Critical Journal Review MK: Bahasa Inggris Fisika Prodi S1 Pendidikan FisikaDocument4 pagesCritical Journal Review MK: Bahasa Inggris Fisika Prodi S1 Pendidikan FisikaBatak CyberTeamNo ratings yet
- Affleck Dine MechanismDocument38 pagesAffleck Dine MechanismAshla MuadNo ratings yet
- Lang Muir 1918Document43 pagesLang Muir 1918Camila NevesNo ratings yet
- States of Matter - Part II. The Three Additional States: Plasma, Bose-Einstein Condensate and Fermionic CondensateDocument6 pagesStates of Matter - Part II. The Three Additional States: Plasma, Bose-Einstein Condensate and Fermionic CondensateCRIZ CATIBOGNo ratings yet
- A Study On Black-Body Radiation Classical and Binary PhotonsDocument20 pagesA Study On Black-Body Radiation Classical and Binary PhotonsSS2005No ratings yet
- Bohm FeynmanDocument15 pagesBohm FeynmanGrecia OjedaNo ratings yet
- Aharanov - Bohm - Mónaco Et AlDocument20 pagesAharanov - Bohm - Mónaco Et AlLizNo ratings yet
- Boltzmann Constant Wiki CCDocument8 pagesBoltzmann Constant Wiki CCjammer999No ratings yet
- Article 2 yDocument11 pagesArticle 2 yrune siqueiraNo ratings yet
- MassDocument4 pagesMassIsfandayaarNo ratings yet
- Chem Entry #4Document67 pagesChem Entry #4Vivialyn YumulNo ratings yet
- Elementary Particles: The Commonwealth and International LibraryFrom EverandElementary Particles: The Commonwealth and International LibraryNo ratings yet
- QSP Chapter7-BoltzmanasdDocument16 pagesQSP Chapter7-Boltzmanasdmasoud0% (1)
- Brownian Motion in Non-Equilibrium Systems and The Ornstein-Uhlenbeck Stochastic ProcessDocument7 pagesBrownian Motion in Non-Equilibrium Systems and The Ornstein-Uhlenbeck Stochastic ProcessTruong Ngoc CuongNo ratings yet
- AP Chemistry - Important Scientists and Their ContributionsDocument4 pagesAP Chemistry - Important Scientists and Their ContributionsJester AlobNo ratings yet
- Jurnal Tentang Mekanika StatistikDocument5 pagesJurnal Tentang Mekanika StatistikRo RohadiNo ratings yet
- PTP 124 731Document16 pagesPTP 124 731Kalakkan KollamNo ratings yet
- Fluctuation PhenomenaFrom EverandFluctuation PhenomenaE MontrollNo ratings yet
- The Principle of Relativity ( Original Papers) by Albert Einstein and Hermann MinkowskiFrom EverandThe Principle of Relativity ( Original Papers) by Albert Einstein and Hermann MinkowskiNo ratings yet









![[Cambridge Mathematical Library] Sydney Chapman, T. G. Cowling, C. Cercignani - The mathematical theory of non-uniform gases_ an account of the kinetic theory of viscosity, thermal conduction, and diffusion in gases (1995, Cambrid.pdf](https://imgv2-1-f.scribdassets.com/img/document/459397571/149x198/a410b3c389/1588373836?v=1)