Professional Documents
Culture Documents
Unique TB-HIV Research Institute Planned in South Africa
Uploaded by
arijadneotteOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Unique TB-HIV Research Institute Planned in South Africa
Uploaded by
arijadneotteCopyright:
Available Formats
news
Unique TB-HIV research institute planned in South Africa
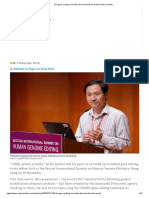
At a time when the dangers of HIV and tuberculosis (TB) co-infection are increasingly apparent, the Howard Hughes Medical Institute (HHMI) has teamed up with the University of KwaZulu-Natal in South Africa, to create a new international research facility dedicated entirely to studying these two diseases, as well as how they interact. The KwaZulu-Natal Research Institute for Tuberculosis and HIV will be housed within the grounds of the Nelson Mandela School of Medicine in Durban. There is no place in the world where there is a dedicated integrated TB-HIV research center, so this is really the first one of those. And what I think is so exciting about this is that it is right in the middle of the worst parts of those epidemics, says Bruce Walker, an HHMI investigator who will lead immunology research within the HIV program at the new KwaZuluNatal Research Institute. The region is ravaged by one of the worlds most devastating TB-HIV co-epidemics, with about half of the local population suffering from HIV/AIDS. It is in the provinces rural area of Tugela Ferry, where a severe outbreak of extremely drug-resistant TB was reported in 2006. Construction of the new institute, a six-story building that will contain high-security labs for TB research, is scheduled to begin in September. In addition to providing a platform for testing
2009 Nature America, Inc. All rights reserved.
A need for information: TB diagnostics is one of the areas the center will study
new vaccines and drugs, it is designed to serve as a global resource to attract top talent and train the next generation of African scientists. There are vanishingly few opportunities for foreigntrained African researchers to come back and do research in their country, says Walker. HHMI has promised funding worth $60 million over the next ten years to secure the long-term vision of the key HHMI scientists involved.
This is a huge investment by the Howard Hughes Medical Institute to go out and do, and it is entirely unprecedented for them to move into this direction, says William Jacobs, Jr. of the Albert Einstein College of Medicine in New York. Jacobs, an HHMI investigator, will offer expertise for the KwaZulu-Natal Research Institute in the development of rapid diagnostic tests for TB. Karen Dente, Paris
Coast IRB hits treacherous waters
In early March, Coast IRB, a company based in Colorado Springs, Colorado that offers independent review board services for clinical trials, issued an unusual pair of press releases. The first claimed that the company had uncovered an inappropriate clinical trial and immediately alerted the authorities. A few days later, Coast reported that the inappropriate trial was actually part of a sting operation run by the US Government Accountability Office (GAO). Some journalists, including this writer, commented in news stories and blogs on what appeared to be an amusing but ultimately harmless chain of events: the GAO probed IRBs, and Coast apparently passed the test. But, according to government investigators, Coast had in fact granted an expedited approval to the fake clinical trial, revoking it five months later only after being contacted by congressional investigators. Coast IRB, a for-profit company, approved a fictitious study led by a fictitious doctor and submitted by a fictitious company, stated US House Oversight and Investigations Subcommittee chairman Bart Stupak at a 26 March hearing on the matter. Worse, the GAOs sham study had been rejected up front by two other IRBs, who had slammed the protocol as being too risky. It was not Coasts first run-in with the authorities. Precisely a year earlier, the US Food and Drug Administration (FDA) had temporarily suspended the companys ability to perform expedited trial reviews, after ruling that Coast had improperly approved a trials recruitment poster. Company representatives remained on the offensive, at least initially. In his opening statement, Coast chief executive officer Daniel Dueber asserted that the Government Accountability Office, at the behest of this Committee, perpetrated an extensive fraud against my company, Coast IRB. It did so without probable cause that Coast had committed any crime. Indeed, no one at Coast has committed any crime. Dueber went on to claim that the GAOs sting operation had violated state and federal law to prove a political point. After a thorough grilling by the committee, however, Dueber abruptly changed tacks. In a contrite statement issued shortly after the hearing, he announced a barrage of policy changes at Coast IRB and conceded that we were duped. Had we started from a premise of skepticism rather than trust, we would not have been. And in a major development two weeks later, the FDA announced on 14 April that Coast IRB had agreed to voluntarily freeze ongoing reviews of clinical trials at the request of the agencya move the FDA said could affect more than 300 trials.
Alan Dove, Springfield, Massachusetts
470
volume 15 | number 5 | may 2009 nature medicine
Associated Press
You might also like
- Agenda 21 Exposed!: The Demolition of Freedom through the Green Deal & The Great Reset 2021-2030-2050 Plandemic - Economic Crisis - HyperinflationFrom EverandAgenda 21 Exposed!: The Demolition of Freedom through the Green Deal & The Great Reset 2021-2030-2050 Plandemic - Economic Crisis - HyperinflationNo ratings yet
- Cases of Scientific MisconductsDocument11 pagesCases of Scientific MisconductsPau BorlagdanNo ratings yet
- Prominent Huntington's Disease Researcher "Fabricated Data" in Grant ApplicationsDocument2 pagesProminent Huntington's Disease Researcher "Fabricated Data" in Grant ApplicationsannaNo ratings yet
- Gov Grisham Letter COVIDDocument6 pagesGov Grisham Letter COVID1SantaFeanNo ratings yet
- Ricks Domestic TerrorismDocument6 pagesRicks Domestic TerrorismFrancis MummaNo ratings yet
- The History of Zidovudine (AZT) : Partnership and ConflictDocument12 pagesThe History of Zidovudine (AZT) : Partnership and ConflictMark Yarchoan100% (3)
- COVID19 Estate LetterDocument22 pagesCOVID19 Estate Letter:Nikolaj: Færch.100% (1)
- Everything Is A Lie - Disinformation CampaignDocument11 pagesEverything Is A Lie - Disinformation CampaignEyemanProphetNo ratings yet
- The Lancet Hydroxychloroquine FraudDocument10 pagesThe Lancet Hydroxychloroquine FraudCK_2023100% (1)
- So You Gave Up All Your Rights, Freedoms and Bodily Autonomy Over A Virus With A 1 Percent Mortality RateDocument13 pagesSo You Gave Up All Your Rights, Freedoms and Bodily Autonomy Over A Virus With A 1 Percent Mortality RatePsychotic100% (3)
- HIV and TuberculosisDocument4 pagesHIV and TuberculosisjsbretingerNo ratings yet
- Cohen India Slashes EstimateDocument2 pagesCohen India Slashes EstimatemakmgmNo ratings yet
- Covid-19 Bio-Warfare and The 21ST Century PandemicDocument9 pagesCovid-19 Bio-Warfare and The 21ST Century PandemicjavenacostaNo ratings yet
- Everything Is A LieDocument35 pagesEverything Is A Liebigwill35No ratings yet
- CIA Torture Appears To Have Broken Spy Agency Rule On Human Experimentation - US News - The Guardian-4Document4 pagesCIA Torture Appears To Have Broken Spy Agency Rule On Human Experimentation - US News - The Guardian-4sagor sagorNo ratings yet
- Cornovirus PDFDocument61 pagesCornovirus PDFWillie Johnson100% (1)
- Immunity Cards FPR AmericansDocument2 pagesImmunity Cards FPR AmericansWillie JohnsonNo ratings yet
- N.I.H. Says Bat Research Group Failed To Submit Prompt Virus Findings - The New York TimesDocument4 pagesN.I.H. Says Bat Research Group Failed To Submit Prompt Virus Findings - The New York TimesCesar Augusto CarmenNo ratings yet
- Authentic Reading - Animal TestingDocument4 pagesAuthentic Reading - Animal TestingTran Anh TuanNo ratings yet
- Letter To Senate Detailing NIH, NIAID and FDA MalfeasanceDocument7 pagesLetter To Senate Detailing NIH, NIAID and FDA MalfeasanceMichael Suede100% (2)
- How Does The Coronavirus Test Work? 5 Questions AnsweredDocument10 pagesHow Does The Coronavirus Test Work? 5 Questions AnsweredAdalberto Caldeira Brant FilhoNo ratings yet
- Health Alerts (10 25 2009)Document14 pagesHealth Alerts (10 25 2009)DrScottJohnsonTeachingsNo ratings yet
- The California HIV/AIDS Research Program: History, Impact, and HIV Cure InitiativeDocument5 pagesThe California HIV/AIDS Research Program: History, Impact, and HIV Cure InitiativeAyuNo ratings yet
- SMALL BUSINESS Learning Entrepreneurship The U.S. Way at M.I.TDocument3 pagesSMALL BUSINESS Learning Entrepreneurship The U.S. Way at M.I.TSecluded ShashiNo ratings yet
- First Case of U.K. Variant of The Coronavirus Detected in Colorado - The Washington PostDocument4 pagesFirst Case of U.K. Variant of The Coronavirus Detected in Colorado - The Washington PostАртём ЗотинNo ratings yet
- At Medical MicrobesDocument30 pagesAt Medical MicrobesBeauNo ratings yet
- COVID Variant Spreads To More Countries As World On Alert - AP NewsDocument5 pagesCOVID Variant Spreads To More Countries As World On Alert - AP NewsMattNo ratings yet
- Preemptive SARS PatentsDocument3 pagesPreemptive SARS PatentssylodhiNo ratings yet
- Forensic Science Reform: Protecting the InnocentFrom EverandForensic Science Reform: Protecting the InnocentWendy J KoenNo ratings yet
- U.S. Asks JournalsDocument4 pagesU.S. Asks JournalsVera MillerNo ratings yet
- Differnence in Med Procedures - 2Document6 pagesDiffernence in Med Procedures - 2api-642960909No ratings yet
- Autism and MMR Vaccine Study BMJDocument5 pagesAutism and MMR Vaccine Study BMJMichelle UrmanNo ratings yet
- Chinese Crime of The CenturyDocument2 pagesChinese Crime of The CenturyYogesh BaggaNo ratings yet
- A Brief Timeline of The Wuhan Lab Dr. Fauci FundedDocument13 pagesA Brief Timeline of The Wuhan Lab Dr. Fauci FundedNguyễn Nhung100% (1)
- 2008 Annas BioterrorBioarteDocument6 pages2008 Annas BioterrorBioarteENo ratings yet
- News ReportDocument1 pageNews ReportGusti Lanang SaputraNo ratings yet
- A Brilliant Scientist Was Mysteriously Fired From A Winnipeg Virus Lab. No One Knows Why. - Macleans - CaDocument12 pagesA Brilliant Scientist Was Mysteriously Fired From A Winnipeg Virus Lab. No One Knows Why. - Macleans - CapNo ratings yet
- Wuhan Complaint InterceptDocument31 pagesWuhan Complaint InterceptRoberto MangioneNo ratings yet
- The Gene Editing Revelation That Shocked The WorldDocument5 pagesThe Gene Editing Revelation That Shocked The Worldfaithme googleNo ratings yet
- When Patti Murphy Came Down With COVIDDocument3 pagesWhen Patti Murphy Came Down With COVIDDaniela SimonelliNo ratings yet
- The Great COVID Cover-UpsDocument9 pagesThe Great COVID Cover-UpsMikeCNo ratings yet
- FDA Enforcement of Criminal Liability For Clinical Investigator FDocument33 pagesFDA Enforcement of Criminal Liability For Clinical Investigator FCherrybelle ParuchelNo ratings yet
- Letter To White House Officials Feb 10 2020Document12 pagesLetter To White House Officials Feb 10 2020All News Pipeline100% (3)
- Regulators Confront Blind Spots in Research Oversight: Double Trouble: An Algorithm Finds DuplicationDocument1 pageRegulators Confront Blind Spots in Research Oversight: Double Trouble: An Algorithm Finds DuplicationarijadneotteNo ratings yet
- My Big WHAT IFDocument3 pagesMy Big WHAT IFAngelou BacolodNo ratings yet
- Watching What We Flush Could Help Keep A Pandemic Under Control - The New York TimesDocument3 pagesWatching What We Flush Could Help Keep A Pandemic Under Control - The New York TimesOnurNo ratings yet
- Nursingupdate TBDocument2 pagesNursingupdate TBJezelle SevillaNo ratings yet
- Wnsum22 Part 58Document1 pageWnsum22 Part 58Holiday GreetingsNo ratings yet
- PCRM President Neal Barnard MD Fails To Answer Why He Provided False Information To Blogger, 4/16/10Document4 pagesPCRM President Neal Barnard MD Fails To Answer Why He Provided False Information To Blogger, 4/16/10deanNo ratings yet
- Key Events in Ethical ResearchDocument6 pagesKey Events in Ethical Researcharhodes777No ratings yet
- Cara Christ Resignation PetitionDocument26 pagesCara Christ Resignation PetitionJoshua MoralesNo ratings yet
- Science, Leadership, and Public Trust in The COVID-19 PandemicDocument5 pagesScience, Leadership, and Public Trust in The COVID-19 PandemicEve AthanasekouNo ratings yet
- Major Testing Issues in US - 2020 - New ScientistDocument1 pageMajor Testing Issues in US - 2020 - New ScientistizmiNo ratings yet
- The Truth About Hydrazine SulfateDocument4 pagesThe Truth About Hydrazine SulfateMichael FlynnNo ratings yet
- Circumcision Hailed As Way To Curb AidsDocument4 pagesCircumcision Hailed As Way To Curb AidsNabeelNo ratings yet
- No Jab For Me: Did You Know?Document13 pagesNo Jab For Me: Did You Know?Jamie RobinsonNo ratings yet
- Human Genome Discoveries Reach The BedsideDocument12 pagesHuman Genome Discoveries Reach The BedsideireneNo ratings yet
- Icc-Complaint-7 (1) Filed by Hannah RoseDocument45 pagesIcc-Complaint-7 (1) Filed by Hannah RoseDuncan Kershaw100% (3)
- Relapse of Cured HIV PatientDocument2 pagesRelapse of Cured HIV PatientMatin Ahmad KhanNo ratings yet
- Tele-Medicine: Presented by Shyam.s.s I Year M.SC NursingDocument12 pagesTele-Medicine: Presented by Shyam.s.s I Year M.SC NursingShyamNo ratings yet
- IELTS Materials ReadingDocument9 pagesIELTS Materials ReadingßläcklìsètèdTȜè0% (1)
- Buzan, Barry - Security, The State, The 'New World Order' & BeyondDocument15 pagesBuzan, Barry - Security, The State, The 'New World Order' & Beyondyossara26100% (3)
- Angelic Spirit Work in The Hoodoo TraditionDocument6 pagesAngelic Spirit Work in The Hoodoo TraditionDaniel Sampaio100% (1)
- Blaine Ray HandoutDocument24 pagesBlaine Ray Handoutaquilesanchez100% (1)
- 13-Mike Engelbrecht - Methods of Maintenance On High Voltage Fluid FilledDocument5 pages13-Mike Engelbrecht - Methods of Maintenance On High Voltage Fluid FilledRomany AllamNo ratings yet
- Unpriced Proposed Renovation of Bugolobi Flat, Block C For Uganda Coffee Development AuthorityDocument39 pagesUnpriced Proposed Renovation of Bugolobi Flat, Block C For Uganda Coffee Development AuthoritynicolasNo ratings yet
- Report Text The Duck Billed Platypus: (Ornithorhynchus Anatinus)Document2 pagesReport Text The Duck Billed Platypus: (Ornithorhynchus Anatinus)Lilis IndriyaniNo ratings yet
- ScriptDocument12 pagesScriptWaleed Nadeem50% (2)
- Sector San Juan Guidance For RepoweringDocument12 pagesSector San Juan Guidance For RepoweringTroy IveyNo ratings yet
- Somanabolic+Muscle+Maximizer+PDF+ +eBook+Free+Download+Kyle+LeonDocument34 pagesSomanabolic+Muscle+Maximizer+PDF+ +eBook+Free+Download+Kyle+LeonAaron BarclayNo ratings yet
- Selling AIESEC To Your TargetsDocument7 pagesSelling AIESEC To Your TargetspijoowiseNo ratings yet
- MGMT 4Document26 pagesMGMT 4Said GunayNo ratings yet
- Calculating Periodic Returns and Compound Annual ReturnsDocument2 pagesCalculating Periodic Returns and Compound Annual ReturnsAlucard77777No ratings yet
- VisualizationDocument2 pagesVisualizationKIRAN H SNo ratings yet
- E650E650M-17 Guía Estándar para El Montaje de Sensores Piezoeléctricos de Emisión Acústica1Document4 pagesE650E650M-17 Guía Estándar para El Montaje de Sensores Piezoeléctricos de Emisión Acústica1fredy lopezNo ratings yet
- Education and Its LegitimacyDocument4 pagesEducation and Its LegitimacySheila G. Dolipas100% (6)
- Data Processing and Management Information System (AvtoBərpaEdilmiş)Document6 pagesData Processing and Management Information System (AvtoBərpaEdilmiş)2304 Abhishek vermaNo ratings yet
- Binary To DecimalDocument8 pagesBinary To DecimalEmmanuel JoshuaNo ratings yet
- A Child With Fever and Hemorrhagic RashDocument3 pagesA Child With Fever and Hemorrhagic RashCynthia GNo ratings yet
- Kibera Mirror JULYDocument8 pagesKibera Mirror JULYvincent achuka maisibaNo ratings yet
- Xiameter OFS-6020 Silane: Diaminofunctional Silane Features ApplicationsDocument2 pagesXiameter OFS-6020 Silane: Diaminofunctional Silane Features ApplicationsDelovita GintingNo ratings yet
- Repeater Panel User GuideDocument24 pagesRepeater Panel User Guideamartins1974No ratings yet
- 5 Waves AnswersDocument2 pages5 Waves AnswersNoor Ulain NabeelaNo ratings yet
- Three Moment Equation For BeamsDocument12 pagesThree Moment Equation For BeamsRico EstevaNo ratings yet
- SFC PresentationDocument51 pagesSFC PresentationjmtriggerzNo ratings yet
- Newsite KPI Check. - Ver2Document4,183 pagesNewsite KPI Check. - Ver2nasircugaxNo ratings yet
- PGT Computer Science Kendriya Vidyalaya Entrance Exam Question PapersDocument117 pagesPGT Computer Science Kendriya Vidyalaya Entrance Exam Question PapersimshwezNo ratings yet
- Altium Designer Training For Schematic Capture and PCB EditingDocument248 pagesAltium Designer Training For Schematic Capture and PCB EditingAntonio Dx80% (5)
- Arch Plan-Agner Boco (For Blue Print) - p1Document1 pageArch Plan-Agner Boco (For Blue Print) - p1Jay CeeNo ratings yet