Professional Documents
Culture Documents
Trend in Melting Points of Group 2 Elements
Trend in Melting Points of Group 2 Elements
Uploaded by
Dominic TamOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Trend in Melting Points of Group 2 Elements
Trend in Melting Points of Group 2 Elements
Uploaded by
Dominic TamCopyright:
Available Formats
Trend in melting points of Group 2 elements
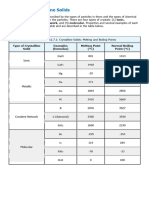
The melting point generally decreases going down Group 2. Table of physical data Proton Melting point Symbol number (K) beryllium 4 Be 1551 magnesium 12 Mg 922 calcium 20 Ca 1112 strontium 38 Sr 1042 barium 56 Ba 998 Element Graph of physical data
Explanation of this trend The Group 2 elements are all metals with metallic bonding, so you expect their melting points to be high. In metallic bonding, metal cations in a metal lattice are attracted to delocalised electrons. Going down Group 2: the number of delocalised electrons remains the same ... the charge on each metal cation stays the same at 2+, but ... the ionic radius increases ... so the attraction between the delocalised electrons and the metal cations decreases.
Notice that, although in general the melting point decreases going down the group, the melting point for magnesium is anomalously low. One possible explanation is that beryllium and magnesium have different metallic structures from the other elements in the group: beryllium and magnesium have a hexagonal close-packed structure; calcium and strontium have a face-centred cubic structure; and barium has a body-centred cubic structure. Copyright 2003 Nigel Saunders N-ch1-28
You might also like
- 20 - Periodic Table - Group 4Document3 pages20 - Periodic Table - Group 4winnielong100% (1)
- Periodicity HL: Ib DP ChemistryDocument61 pagesPeriodicity HL: Ib DP ChemistryLaura Estefania MoraNo ratings yet
- 2.7 The Periodic Table - Groups 2 and 7Document84 pages2.7 The Periodic Table - Groups 2 and 7Listiyaning TiasNo ratings yet
- Group 2the Alkaline Earth MetalsDocument24 pagesGroup 2the Alkaline Earth Metalsmadeee92No ratings yet
- FaziraRazak Group IIADocument58 pagesFaziraRazak Group IIAaieyinHengNo ratings yet
- Chemistry STPM Semester 2 Group 2Document7 pagesChemistry STPM Semester 2 Group 2kumutha83% (6)
- Group 2Document32 pagesGroup 2irnihafizan6812No ratings yet
- Chemistry Form 6 Sem 2 04 Notes STPM 2014/2013Document27 pagesChemistry Form 6 Sem 2 04 Notes STPM 2014/2013Raj Nittiya SugumaranNo ratings yet
- Trends in Group 2 Elements Alkaline Earth MetalsDocument52 pagesTrends in Group 2 Elements Alkaline Earth MetalsKemoy FrancisNo ratings yet
- Trends in Group 2 Elements (Alkaline Earth Metals)Document52 pagesTrends in Group 2 Elements (Alkaline Earth Metals)Antonique HeadmanNo ratings yet
- Group 2 MetalsDocument19 pagesGroup 2 MetalsSelena JayyNo ratings yet
- Pioneer Junior College H2 CHEMISTRY (9647) Group II: ReferencesDocument11 pagesPioneer Junior College H2 CHEMISTRY (9647) Group II: ReferencesTimothy HandokoNo ratings yet
- Chemistry STPM Semester 2 Group 2Document12 pagesChemistry STPM Semester 2 Group 2Chong Yin Ping100% (1)
- Group 2 Notes (Sem 2)Document7 pagesGroup 2 Notes (Sem 2)Geethanjali SivakumarNo ratings yet
- Group 2Document31 pagesGroup 2Shima SenseiiNo ratings yet
- The S-Block ElementsDocument9 pagesThe S-Block ElementsKamal DeshapriyaNo ratings yet
- Chapter 2: Group 2A Metals 1. Call The Name of The Elements?Document10 pagesChapter 2: Group 2A Metals 1. Call The Name of The Elements?Phượng NguyễnNo ratings yet
- Chapter 10 Group 2Document8 pagesChapter 10 Group 2Vjayan DharmaNo ratings yet
- 2013 Group II Lecture Notes (Student) .UnlockedDocument20 pages2013 Group II Lecture Notes (Student) .UnlockedKwok Yee TherNo ratings yet
- Group 2 - The Alkaline Earth Metals: AppearanceDocument5 pagesGroup 2 - The Alkaline Earth Metals: AppearanceLourdes Nitro MarasiganNo ratings yet
- 11 Group 2 and 17 (S)Document6 pages11 Group 2 and 17 (S)Mr TanNo ratings yet
- ChemDocument20 pagesChemMarcellePierreNo ratings yet
- S BLOCK Elements JeeDocument155 pagesS BLOCK Elements JeeSaahil JainNo ratings yet
- Publication 1 1129 24Document14 pagesPublication 1 1129 24Marwan FarhanNo ratings yet
- Group Two-The Alkaline Earth MetalsDocument51 pagesGroup Two-The Alkaline Earth MetalsAdegoke TofunmiNo ratings yet
- 11 - Group 2Document37 pages11 - Group 2enderothNo ratings yet
- Chemical Periodicity (REVISED)Document3 pagesChemical Periodicity (REVISED)Annie Valerie OgedeNo ratings yet
- Group 2 Elements: UNIT 1: MOD 3 2.1-2.5Document17 pagesGroup 2 Elements: UNIT 1: MOD 3 2.1-2.5Ninti BraithwaiteNo ratings yet
- Topic 1 The S Block Elements Group 1 2 Part 1Document34 pagesTopic 1 The S Block Elements Group 1 2 Part 1A/P SUPAYA SHALININo ratings yet
- 10.1 Group 2Document43 pages10.1 Group 2safiya_91No ratings yet
- Pblock NotesDocument94 pagesPblock NotesSparshNo ratings yet
- Group Ii Elements: S-Block Elements Because Their Valence (Bonding) Electrons Are in S OrbitalsDocument3 pagesGroup Ii Elements: S-Block Elements Because Their Valence (Bonding) Electrons Are in S OrbitalsAndreea Maria PavelNo ratings yet
- Cambridge Book Group 2Document8 pagesCambridge Book Group 2Aree WonNo ratings yet
- Helpful For CAPE U1 Chemistry Transition Elements PDFDocument30 pagesHelpful For CAPE U1 Chemistry Transition Elements PDFXia U Rong100% (1)
- D-Block ElementDocument15 pagesD-Block ElementFedex WalterNo ratings yet
- Classes of Crystalline Solids 4 Types of CrystalDocument5 pagesClasses of Crystalline Solids 4 Types of Crystaldellrubion011No ratings yet
- Chemistry Module 2 Application If Metal ComplexesDocument56 pagesChemistry Module 2 Application If Metal ComplexesRiyazNo ratings yet
- PhaseIII Chem L5 ReactionOfMetalsDocument3 pagesPhaseIII Chem L5 ReactionOfMetalsBHAKTI YOGANo ratings yet
- Topic 2 - ComplexationDocument48 pagesTopic 2 - ComplexationLokesh JaiswalNo ratings yet
- Module 2 - Metal Complexes and OrganometallicsDocument55 pagesModule 2 - Metal Complexes and Organometallicstaara022006No ratings yet
- Group II ElementsDocument15 pagesGroup II ElementsDoveNo ratings yet
- 8B Group 1 2Document14 pages8B Group 1 2pediaNo ratings yet
- Group Iia ElementsDocument27 pagesGroup Iia ElementsYaboi MattNo ratings yet
- Lesson 1Document19 pagesLesson 1saidbiala414No ratings yet
- Periodicity (ANNEX) - CN - STDT3Document2 pagesPeriodicity (ANNEX) - CN - STDT3NkemziNo ratings yet
- Chemistry Module 2 Part 2Document60 pagesChemistry Module 2 Part 2RiyazNo ratings yet
- New Module-2 Inorganic and Organometallic Chem Fall-2023Document67 pagesNew Module-2 Inorganic and Organometallic Chem Fall-2023VICHUNo ratings yet
- Module-2-Dr RKDocument55 pagesModule-2-Dr RKIshaan SawantNo ratings yet
- Akaline Earth Metals - Group 2 NotesDocument5 pagesAkaline Earth Metals - Group 2 NotesQweemyah IsraelNo ratings yet
- Anomalous Behaviour of BerylliumDocument8 pagesAnomalous Behaviour of BerylliumSatyrKuangNo ratings yet
- Group I and II ElementsDocument33 pagesGroup I and II ElementsMompoloki Bluda GabathusiNo ratings yet
- Intro To Transition Metal Complexes (CH 21)Document4 pagesIntro To Transition Metal Complexes (CH 21)Husham HussanNo ratings yet
- Periodic TableDocument41 pagesPeriodic TableNusrat'Felix' KhanNo ratings yet
- Science-Grade-9-Handout-2-Ion Formation and LEDSDocument10 pagesScience-Grade-9-Handout-2-Ion Formation and LEDSClinton YmbongNo ratings yet
- Subject: Chemistry Class: XI Chapter: The S-Block Elements Top ConceptsDocument10 pagesSubject: Chemistry Class: XI Chapter: The S-Block Elements Top ConceptsRISHI KEJRIWALNo ratings yet
- Wa0008.Document24 pagesWa0008.Meenakshi SuhagNo ratings yet
- Group 2 ElementsDocument39 pagesGroup 2 ElementsSIVANESVARANNo ratings yet
- Endohedral Metallofullerenes: Fullerenes with Metal InsideFrom EverandEndohedral Metallofullerenes: Fullerenes with Metal InsideNo ratings yet