Professional Documents
Culture Documents
Aromatic Halogen
Aromatic Halogen
Uploaded by
Ashila NurainiOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Aromatic Halogen
Aromatic Halogen
Uploaded by
Ashila NurainiCopyright:
Available Formats
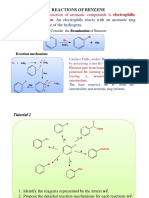
Halogenation of Benzene
Reaction type: Electrophilic Aromatic Substitution
Summary.
Overall transformation : Ar-H to Ar-X Reagent : normally the halogen (e.g. Br2) with a Lewis acid catalyst The active catalyst is not Fe (0) but the FeX3 formed by reaction of Fe with X2 Electrophilic species : the halonium ion (i.e. X +) formed by the removal of a halide ion by the Lewis acid catalyst Restricted to Cl2 and Br2. I- or F- are usually introduced using alternative methods
MECHANISM FOR HALOGENATION OF BENZENE Step 1: The bromine reacts with the Lewis acid to form a complex that makes the bromine more electrophilic. Step 2: The electrons of the aromatic C=C act as a nucleophile, attacking the electrophilic Br, and displacing iron tetrabromide. This step destroys the aromaticity giving the cyclohexadienyl cation intermediate.
Step 3: Removal of the proton from the sp3 C bearing the bromo- group reforms the C=C and the aromatic system, generating HBr and regenerating the active catalyst.
You might also like
- Electrophilic Aromatic Substitution + The Chemistry of BenzeneDocument28 pagesElectrophilic Aromatic Substitution + The Chemistry of BenzeneTrescia Mae EstilloreNo ratings yet
- Regioselective Reactions: Regioselective Means That The Reaction Selectively Produces One Regioisomer As TheDocument8 pagesRegioselective Reactions: Regioselective Means That The Reaction Selectively Produces One Regioisomer As Therihana yadavNo ratings yet
- Aromatic Compound Theory - EDocument23 pagesAromatic Compound Theory - Ethinkiit100% (1)
- Electrophilic Addition of Alkenes NotesDocument17 pagesElectrophilic Addition of Alkenes NotesAnanda Vijayasarathy100% (1)
- Organic Chemistry B Q BNK AnswerDocument20 pagesOrganic Chemistry B Q BNK AnswerAqueel HusainNo ratings yet
- Chemistry Form 6 Sem 3 03Document39 pagesChemistry Form 6 Sem 3 03Ng Swee Loong StevenNo ratings yet
- Chap 16 (Notes)Document134 pagesChap 16 (Notes)Steffi YapNo ratings yet
- Exercises 6,7,8 HandoutDocument162 pagesExercises 6,7,8 HandoutErvi Festin PangilinanNo ratings yet
- ArenesDocument5 pagesArenes林琪No ratings yet
- Chemistry Form 6 Sem 3 Chapter 3Document39 pagesChemistry Form 6 Sem 3 Chapter 3Yuzamrah Awang NohNo ratings yet
- Aromatic Substitution PDFDocument13 pagesAromatic Substitution PDFNesha VincentNo ratings yet
- Electrophile: Organic ChemistryDocument7 pagesElectrophile: Organic ChemistryJesse SawianNo ratings yet
- The Most Common Reaction of Aromatic Compounds Is ElectrophilicDocument2 pagesThe Most Common Reaction of Aromatic Compounds Is ElectrophilicPietNo ratings yet
- Chapter 11. Free Radical ReactionsDocument7 pagesChapter 11. Free Radical ReactionsGilang HerjunaNo ratings yet
- Class 12 Organic SupplementsDocument14 pagesClass 12 Organic Supplementslovishsindhi185No ratings yet
- Properties of HydrocarbonDocument9 pagesProperties of Hydrocarbon刘象100% (1)
- HaloalkanesDocument13 pagesHaloalkanesChingYan TanNo ratings yet
- Reactions of Benzene and Alkylbenzene A Level A2 Chemistry CIEDocument7 pagesReactions of Benzene and Alkylbenzene A Level A2 Chemistry CIErayaNo ratings yet
- Chapter 15 NotesDocument5 pagesChapter 15 NotesNicole BercegeayNo ratings yet
- Alkene Synthesis & ReactionsDocument11 pagesAlkene Synthesis & ReactionsStanley ChuNo ratings yet
- Chapter 16b (AS-Level) : Physical Properties of AlkenesDocument7 pagesChapter 16b (AS-Level) : Physical Properties of AlkenesTilak K CNo ratings yet
- Lecture 2 - Reactions of Aromatic CompoundsDocument164 pagesLecture 2 - Reactions of Aromatic CompoundsQutaiba IbrahimNo ratings yet
- AromaticDocument38 pagesAromaticDerrick Maatla MoadiNo ratings yet
- AromaticsDocument70 pagesAromaticsEceDiril100% (1)
- Hydrocarbon Compounds: AromaticDocument49 pagesHydrocarbon Compounds: AromaticUMMU MARDHIAH ABDUL HALIMNo ratings yet
- EAS NotesDocument39 pagesEAS NotesSudhanshu HedaNo ratings yet
- Haloalkanes and Haloarenes NotesDocument10 pagesHaloalkanes and Haloarenes NotesArchanaa PadmavathiNo ratings yet
- Chemistry of Benzene: Electrophilic Aromatic Substitution: Based On Mcmurry'S Organic Chemistry, 6 Edition, Chapter 16Document89 pagesChemistry of Benzene: Electrophilic Aromatic Substitution: Based On Mcmurry'S Organic Chemistry, 6 Edition, Chapter 16Nur Anis HidayahNo ratings yet
- TOPIC 1 AliphaticDocument17 pagesTOPIC 1 AliphaticFATIMAHNo ratings yet
- Haloalkanes and HaloarenesDocument28 pagesHaloalkanes and HaloarenesDevansh TiwaryNo ratings yet
- Organic MaterialDocument15 pagesOrganic MaterialAditya GathwalaNo ratings yet
- 3 ArenesDocument7 pages3 ArenesAnonymous 8VJhV1eI2yNo ratings yet
- ArenesDocument4 pagesArenesNkemzi Elias NzetengenleNo ratings yet
- Organic Chemistry Alkynes ReactionsDocument9 pagesOrganic Chemistry Alkynes ReactionsAnthony KwofieNo ratings yet
- Haloform ReactionDocument10 pagesHaloform ReactionBhuwnesh SharmaNo ratings yet
- Chemistry of Benzene: Electrophilic Aromatic SubstitutionDocument80 pagesChemistry of Benzene: Electrophilic Aromatic Substitution張湧浩100% (1)
- Haloalkanes and HaloarenesDocument8 pagesHaloalkanes and HaloarenesYash RajNo ratings yet
- NItration, Sulfonation and Halogenation PDFDocument3 pagesNItration, Sulfonation and Halogenation PDFSANJAY S SHEKARNo ratings yet
- 2 4-2 5 (Alkenes)Document9 pages2 4-2 5 (Alkenes)Lyana TaylorNo ratings yet
- Electrophilic Addition of Hydrogen HalidesDocument16 pagesElectrophilic Addition of Hydrogen HalidesDk Hazra HadzryaNo ratings yet
- HalogenalkanesDocument22 pagesHalogenalkanesMiguelNo ratings yet
- Alcohols and Phenols (ROH, Functional GRP - OH.)Document24 pagesAlcohols and Phenols (ROH, Functional GRP - OH.)MadhureemaNo ratings yet
- Vollhardt 6e Lecture PowerPoints - Chapter 12Document74 pagesVollhardt 6e Lecture PowerPoints - Chapter 12superfr3shmNo ratings yet
- Aromatic Electrophilic SubstitutionDocument71 pagesAromatic Electrophilic SubstitutionsridharancNo ratings yet
- Electrophilic Aromatic SubstitutionDocument32 pagesElectrophilic Aromatic Substitutionaminah hameedNo ratings yet
- Notes - Alkyl Halides and Aryl HalidesDocument34 pagesNotes - Alkyl Halides and Aryl HalidesDivya MehtaNo ratings yet
- BenzeneDocument8 pagesBenzenePurpleMyself25No ratings yet
- Benzene StructureDocument30 pagesBenzene StructureAnuki PereraNo ratings yet
- Hsslive Xii CH 6 Haloalkanes AnilDocument13 pagesHsslive Xii CH 6 Haloalkanes AnilUnkown HumanNo ratings yet
- H 0 CCL Naoh H So Cyclohexane (Clear Liquid)Document5 pagesH 0 CCL Naoh H So Cyclohexane (Clear Liquid)Mark GirasolNo ratings yet
- Chemistry of Benzene: Electrophilic Aromatic SubstitutionDocument35 pagesChemistry of Benzene: Electrophilic Aromatic SubstitutionDanny Jorge Huicy FernandezNo ratings yet
- HALOALKANES AND HALOARENES-Anil-Hsslive PDFDocument12 pagesHALOALKANES AND HALOARENES-Anil-Hsslive PDFAnwar Hashmi67% (3)
- 4.2 Hydration of AlkenesDocument33 pages4.2 Hydration of AlkenesDawit BirhanuNo ratings yet
- Benzene and Derivatives Members GroupDocument57 pagesBenzene and Derivatives Members GroupHaris KhanNo ratings yet
- Arenes and Phenols PowerpointDocument17 pagesArenes and Phenols PowerpointBABYNATS1992No ratings yet
- Chemical Properties of Alkenes (Q Only)Document15 pagesChemical Properties of Alkenes (Q Only)mawarhanifNo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- Practice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersFrom EverandPractice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersNo ratings yet