Professional Documents
Culture Documents
Polphys-Ss11 Problems1
Uploaded by
Edward Begbaunimushuye ApebendeOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Polphys-Ss11 Problems1
Uploaded by
Edward Begbaunimushuye ApebendeCopyright:
Available Formats
Problem Set 1
Introduction to Polymer Physics, SS 2011, 13.4.10
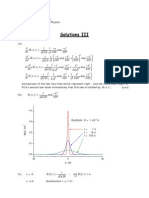
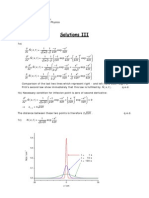
1. Using two polystyrene fractions with very uniform molecular weight distribution (P 1), a bimodal mixture with a mass average molecular weight of Mw = 25000 g/mol and a polydispersity of P = 1.25 should be prepared. The mixture should contain equal numbers of the different chains. Calculate the molecular weight and the number average degree of polymerization of the two components. 2. Step-growth polymerization. (a) Using the equations given in the lecture, prove that the number and weight average 1 degrees of polymerization are given by Nn = 1x (Carothers equation) and Nw = 1+x , 1x respectively (x is the extent of reaction). (b) Comment on the behavior of Nn for x 1. In how far does this make sense physically? What would rather happen in a real system? Why is it hardly possible to ever achieve x = 1, i.e, what are the factors that limit the molecular weight in step-growth reactions? 3. Explain why the intensity of light scattered by a polymer solution depends on the second virial coefcient (A2 ) of the solution. The central argument rests on a question of length scales: How does the wavelength of visible light compare to the average distance between coils (estimate this for a polystyrene solution with Mn = 105 g/mol at 1 g/l, assuming e.g. a cubic arrangement)? Would the polymer fraction (as compared to the pure solvent) scatter any appreciable excess amount of light if the molecules were xed at their positions?
You might also like
- Universal Switch Able RAF AgentsDocument2 pagesUniversal Switch Able RAF AgentsEdward Begbaunimushuye ApebendeNo ratings yet
- LSG PmPhys3Document2 pagesLSG PmPhys3Edward Begbaunimushuye ApebendeNo ratings yet
- LSG PmPhys3Document2 pagesLSG PmPhys3Edward Begbaunimushuye ApebendeNo ratings yet
- Sunthar Polymer RheologyDocument21 pagesSunthar Polymer RheologyPuli IlupNo ratings yet
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (120)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)