Professional Documents
Culture Documents
Carisoprodol
Uploaded by
Brian HarrisOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Carisoprodol
Uploaded by
Brian HarrisCopyright:
Available Formats
Drug Enforcement Administration Office of Diversion Control Drug & Chemical Evaluation Section
CARISOPRODOL
(Trade Name: Soma)
January 2012
DEA/OD/ODE
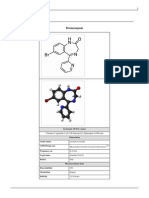
Introduction: Carisoprodol is a prescription drug marketed since 1959. It is a centrally acting muscle relaxant. The diversion and abuse of carisoprodol have increased in the last decade. Licit Uses: Carisoprodol is used as an adjunct to rest, physical therapy and other measures for relief of acute, painful musculoskeletal conditions. It is available as single-entity tablets containing 250 mg or 350 mg carisoprodol, and as combination tablets containing 200 mg carisoprodol, 325 mg aspirin and 16 mg codeine phosphate. The standard dosage for adults is 250 mg to 350 mg three times daily and at bed-time. Use in patients under age 12 is not recommended. According to IMS Health , there were approximately 11.1 million prescriptions for carisoprodol dispensed in 2010 and 2.8 million dispensed in the first quarter of 2011. Chemistry/Pharmacology: Carisoprodol is N-isopropyl-2-methyl-2-propyl-1,3propanediol dicarbamate and is both structurally and pharmacologically related to meprobamate, a schedule IV substance. It has a chemical structure that gives rise to two optical isomers and is typically found as an equal combination of both, which is referred to as a racemic mixture. Carisoprodol does not directly affect skeletal muscle in man. Skeletal muscle relaxant action of carisoprodol may be related to its sedative properties. Recent animal studies conducted under the directive of the National Institute on Drug Abuse (NIDA) indicate that subjective effects of carisoprodol may be similar to other central nervous system depressants such as meprobamate, pentobarbital and chlordiazepoxide and it possesses rewarding effects. These data suggest that carisoprodol has abuse liability. The onset of action of carisoprodol is rapid and effects last 4 to 6 hours. It is metabolized in the liver and excreted through kidney. The major metabolic pathway of carisoprodol involves its conversion to meprobamate, a drug with substantial barbiturate-like biological actions. Adverse reactions may include central nervous system related effects such as drowsiness, dizziness, vertigo, ataxia, tremor, agitation, irritability, head ache, depressive reactions, syncope and insomnia. Carisoprodol may also adversely affect cardiovascular (tachycardia, postural hypotension and facial flushing), gastrointestinal (nausea, vomiting, hiccup and epigastric distress), and hematologic systems. It may cause idiosyncratic symptoms including extreme weakness, transient quadriplegia, ataxia, difficulty in speech, temporary loss of vision, double vision, dilated pupils, agitation, euphoria, confusion, and disorientation. Carisoprodol overdose has resulted in stupor, coma, shock, respiratory depression and death.
Illicit Uses: Carisoprodol abuse has escalated in the last decade in the United States. According to 2009 National Survey on Drug Use and Health (NSDUH) data, 2.95 million people, aged 12 and older, used Soma for non-medical reasons in their lifetime. With prolonged abuse at high dosage, carisoprodol can lead to tolerance, dependence and withdrawal symptoms in humans. Illicit distribution: According to the Diversion Drug Trends, published by the Drug Enforcement Administration (DEA) on the trends in the diversion of controlled and noncontrolled pharmaceuticals, carisoprodol continues to be one of the most commonly diverted drugs. Diversion and abuse of carisoprodol is prevalent throughout the country. As of March 2011, street prices for Soma ranged from $1 to $5 per tablet. Diversion methods include doctor shopping for the purpose of obtaining multiple prescriptions and forging prescriptions. The National Forensic Laboratory Information System (NFLIS) is a DEA database that collects scientifically verified data on drug items and cases submitted to and analyzed by state and local forensic laboratories. The System to Retrieve Information from Drug Evidence provides information on drug seizures reported to and analyzed by DEA laboratories. Substances identified by federal, state and local forensic laboratories, as carisoprodol, increased from 3,988 in 2008 to 5,076 in 2010. In the first quarter of 2011, 870 exhibits were identified as carisoprodol. According to NFLIS, carisoprodol has been consistently in the top 25 most frequently identified drugs by the state and local forensic laboratories since 2000. The American Association of Poison Control Centers reported 8,976 total carisoprodol exposures, 3,685 single exposures and 2 related deaths in 2009. Medical Examiners Commission Reports released by the Florida Department of Law Enforcement (FDLE) indicate that carisoprodol/meprobamate related deaths in Florida increased by more than 100% from 208 in 2003 to 455 in 2009, surpassing opioids such as heroin, fentanyl, and hydromorphone. In the first six months of 2010, 220 deaths were related to carisoprodol/meprobamate. Control status: As of January 11, 2012, Carisoprodol is a schedule IV controlled substance under the Controlled Substances Act of 1970.
Comments and additional information are welcomed by the Drug and Chemical Evaluation Section, Fax 202-353-1263, telephone 202-307-7183, or Email ODE@usdoj.gov.
You might also like
- Nonmedication Treatments For ADHD in AdultsDocument168 pagesNonmedication Treatments For ADHD in AdultsMaricela Virgen Enciso100% (5)
- 6th Central Pay Commission Salary CalculatorDocument15 pages6th Central Pay Commission Salary Calculatorrakhonde100% (436)
- 6th Central Pay Commission Salary CalculatorDocument15 pages6th Central Pay Commission Salary Calculatorrakhonde100% (436)
- Colorado Peace Officer Manual 2013Document373 pagesColorado Peace Officer Manual 2013Brian Harris100% (1)
- Pharmacogenomic and Pharmacogenetics - DIAN PDFDocument34 pagesPharmacogenomic and Pharmacogenetics - DIAN PDFfakultasfarmasiNo ratings yet
- The Psychopharmacology of ParoxetineDocument7 pagesThe Psychopharmacology of ParoxetineolivukovicNo ratings yet
- Bath Salts: Expert Omar Zourob 1Document21 pagesBath Salts: Expert Omar Zourob 1Omar ZourobNo ratings yet
- Steroids Other Appearance Performance Enhancing Drugs Apeds Research ReportDocument34 pagesSteroids Other Appearance Performance Enhancing Drugs Apeds Research ReportFit and LiftNo ratings yet
- Top 100 Drugs in CanadaDocument25 pagesTop 100 Drugs in CanadaMohamed Omer100% (1)
- Drug Interaction of Dental DrugsDocument14 pagesDrug Interaction of Dental DrugsAnubhuti SabhlokNo ratings yet
- IPCRJan June2017Document4 pagesIPCRJan June2017Jenivic Empig Puedan50% (2)
- 2019 April KAPS Exam CompilationDocument9 pages2019 April KAPS Exam CompilationWajihaNo ratings yet
- Black Book Project 3Document69 pagesBlack Book Project 3Pragya SinghNo ratings yet
- Banking - Diksha Book On Effective Study Techniques PDFDocument66 pagesBanking - Diksha Book On Effective Study Techniques PDFHemrajSainiNo ratings yet
- Corticosteroids and Associated DiseasesDocument76 pagesCorticosteroids and Associated DiseasesALNAKINo ratings yet
- Multiple Disability Original HandoutDocument42 pagesMultiple Disability Original HandoutHabtamu DebasuNo ratings yet
- FAMILY NURSING CARE PLAN - AsthmaDocument1 pageFAMILY NURSING CARE PLAN - AsthmaJULIANNE BAYHON80% (5)
- Drug Education and Vice ControlDocument12 pagesDrug Education and Vice ControlJhuan Paulo Aquino ClavecillasNo ratings yet
- In Memoriam David R. Hawkins 27.4Document2 pagesIn Memoriam David R. Hawkins 27.4Aditya VarunNo ratings yet
- People's Vision For MumbaiDocument86 pagesPeople's Vision For MumbaiAravind UnniNo ratings yet
- Global City FinalDocument19 pagesGlobal City FinalPaul Erwin AustriaNo ratings yet
- Introduction To Nursing InformaticsDocument55 pagesIntroduction To Nursing InformaticsRhae Raynog100% (3)
- Carisoprodol-350mg TabletDocument8 pagesCarisoprodol-350mg TabletMd. Abdur RahmanNo ratings yet
- Carisoprodol SomaDocument6 pagesCarisoprodol SomaLuis SantosNo ratings yet
- HaloperidolDocument15 pagesHaloperidolfachsyarNo ratings yet
- Abilify, An Antipsychotic DrugDocument4 pagesAbilify, An Antipsychotic DrugAdam RodriguezNo ratings yet
- Drug Information Bulletin 38 05Document4 pagesDrug Information Bulletin 38 05amritaryaaligarghNo ratings yet
- Section 9 Anti Parkinsonism MedicinesDocument14 pagesSection 9 Anti Parkinsonism MedicinesAmra ahmedNo ratings yet
- ED M 5 PharmacologyDocument12 pagesED M 5 PharmacologyChengDNo ratings yet
- Progressão Do Comprometimento CognitivoDocument21 pagesProgressão Do Comprometimento CognitivoFábio VinholyNo ratings yet
- MG 1210Document8 pagesMG 1210Vivian SutafaNo ratings yet
- Drug & Chemical Evaluation Section: Diversion Control DivisionDocument1 pageDrug & Chemical Evaluation Section: Diversion Control DivisionnataliaNo ratings yet
- Tramadol: (Trade Names: Ultram®, Ultracet®)Document1 pageTramadol: (Trade Names: Ultram®, Ultracet®)Marthaluzy CarinaNo ratings yet
- Lecture 1 IntroductionDocument8 pagesLecture 1 IntroductionManWol JangNo ratings yet
- Psychotropic Drug Quiz 10Document1 pagePsychotropic Drug Quiz 10Jess MontgomeryNo ratings yet
- NIH Public Access: Author ManuscriptDocument10 pagesNIH Public Access: Author ManuscriptHenderaNo ratings yet
- Opdra Postmarketing I Safety Review: Iepartment of Health and Human Ervices Public Hei4.Ll-H ServiceDocument43 pagesOpdra Postmarketing I Safety Review: Iepartment of Health and Human Ervices Public Hei4.Ll-H ServiceMario WilmathNo ratings yet
- Club Drugs (GHB, Ketamine, and Rohypnol)Document4 pagesClub Drugs (GHB, Ketamine, and Rohypnol)tiaraayaaNo ratings yet
- Temp - Please DeleteDocument1 pageTemp - Please DeleteCourier JournalNo ratings yet
- NandraloneDocument24 pagesNandraloneGabriel NguyenNo ratings yet
- Addiction Science - From Molecules To Managed CareDocument75 pagesAddiction Science - From Molecules To Managed Carea1eshNo ratings yet
- Jur Nista TabDocument17 pagesJur Nista Tabnela sharonNo ratings yet
- 254 Homework 2 Metoprolol - Lauren HolveyDocument2 pages254 Homework 2 Metoprolol - Lauren Holveyapi-724192635No ratings yet
- Selected Topics: Toxicology: Severe Carisoprodol Withdrawal After A 14-Year Addiction and Acute OverdoseDocument4 pagesSelected Topics: Toxicology: Severe Carisoprodol Withdrawal After A 14-Year Addiction and Acute OverdoseAhmad SyaukatNo ratings yet
- Anti Psychotics Odt'sDocument26 pagesAnti Psychotics Odt'ssaimanideepakNo ratings yet
- Robaxin-750, 750 MG Film-Coated TabletsDocument4 pagesRobaxin-750, 750 MG Film-Coated TabletsNguyễnMinhTiếnNo ratings yet
- Tramadol: Dana Bartlett, RN, BSN, MSN, MA, CSPIDocument53 pagesTramadol: Dana Bartlett, RN, BSN, MSN, MA, CSPIVette Angelikka Dela CruzNo ratings yet
- FACTBOX-World's Top-Selling Drugs in 2014 Vs 2010: Tue Apr 13, 2010 9:01am EDTDocument10 pagesFACTBOX-World's Top-Selling Drugs in 2014 Vs 2010: Tue Apr 13, 2010 9:01am EDTSucharita MallickNo ratings yet
- DR IsraDocument29 pagesDR IsraMuhammad MasroorNo ratings yet
- Final PPT NSTP Drug Addiction g1Document83 pagesFinal PPT NSTP Drug Addiction g1JovilleNo ratings yet
- Drug Abuse and Its Incidence-3 Different Collective Case - Studies On Acute Iodine Toxicity, Petrol Abuse and Sodium Bicarbonate AbuseDocument6 pagesDrug Abuse and Its Incidence-3 Different Collective Case - Studies On Acute Iodine Toxicity, Petrol Abuse and Sodium Bicarbonate AbuseRAVI JASWANINo ratings yet
- Opioid Withdrawal in The Emergency Setting - UpToDateDocument15 pagesOpioid Withdrawal in The Emergency Setting - UpToDateSanti HerreraNo ratings yet
- TCM PharmacologyDocument3 pagesTCM Pharmacologypj9066No ratings yet
- BuscopanDocument10 pagesBuscopanapi-3755679No ratings yet
- KetorolacDocument8 pagesKetorolacSan LampuyasNo ratings yet
- Ziac (Bisoprolol+HCT)Document21 pagesZiac (Bisoprolol+HCT)San-Clin-Eq LaboratoryNo ratings yet
- Domperidone 1mg - ML Oral Suspension - (eMC) - Print FriendlyDocument7 pagesDomperidone 1mg - ML Oral Suspension - (eMC) - Print FriendlyNanda Asyura RizkyaniNo ratings yet
- Pharmacogenetics Polymorphism and Efficacy of DrugsDocument11 pagesPharmacogenetics Polymorphism and Efficacy of DrugsRohitNo ratings yet
- Psychotropic IntoxicationDocument8 pagesPsychotropic IntoxicationAmit TamboliNo ratings yet
- Lorcaserin Review For The FDA Endocrinologic and Metabolic ACDocument16 pagesLorcaserin Review For The FDA Endocrinologic and Metabolic ACCREWNo ratings yet
- Health Canada - HaloperidolDocument25 pagesHealth Canada - HaloperidolIndrie LestarieNo ratings yet
- Rokacet Rokacet PlusDocument11 pagesRokacet Rokacet PlusNaomie bocobzaNo ratings yet
- 17 05 SPANZA Advisory On Tramadol 31 May 2017 PDFDocument4 pages17 05 SPANZA Advisory On Tramadol 31 May 2017 PDFConstantin StanNo ratings yet
- HaloperidolDocument4 pagesHaloperidolNickol BaylonNo ratings yet
- Research Paper On CodeineDocument6 pagesResearch Paper On Codeineefkm3yz9100% (1)
- Why Propranolol Is Preferred To Other Beta-Blockers in Thyrotoxicosis or Thyroid StormDocument3 pagesWhy Propranolol Is Preferred To Other Beta-Blockers in Thyrotoxicosis or Thyroid StormAnonymous FCOOcnNo ratings yet
- BromazepamDocument6 pagesBromazepamMariusNeicuNo ratings yet
- AAEs and PsychyatricsDocument10 pagesAAEs and PsychyatricsJorge FernándezNo ratings yet
- Steroids Other Appearance Performance Enhancing Drugs Apeds Research ReportDocument35 pagesSteroids Other Appearance Performance Enhancing Drugs Apeds Research ReportSiddhartha SahaNo ratings yet
- EDL Ethiopia 2002Document37 pagesEDL Ethiopia 2002hailebiruadeyalewNo ratings yet
- Assignment ErythropoietinDocument4 pagesAssignment ErythropoietinvikramnmbaNo ratings yet
- Dic 4 33Document4 pagesDic 4 33amritaryaaligarghNo ratings yet
- Colorado Medical Use of Marijuana Revised Statutes 18-18-406.3Document2 pagesColorado Medical Use of Marijuana Revised Statutes 18-18-406.3Brian HarrisNo ratings yet
- Colorado Medical Use of Marijuana Department of Public Health and Environment Health and Environmental Information and Statistics Division 5 CCR 1006-2Document18 pagesColorado Medical Use of Marijuana Department of Public Health and Environment Health and Environmental Information and Statistics Division 5 CCR 1006-2Brian HarrisNo ratings yet
- Colorado Revised Statutes (CRS 12 42.5) Title 12 Article 42.5 Professions and Occupations Pharmacists, Pharmacy Businesses, and PharmaceuticalsDocument48 pagesColorado Revised Statutes (CRS 12 42.5) Title 12 Article 42.5 Professions and Occupations Pharmacists, Pharmacy Businesses, and PharmaceuticalsBrian HarrisNo ratings yet
- Colorado CRS 18 18 - Uniform Controlled Substances Acto of 1992 Effective 7-1-2009Document69 pagesColorado CRS 18 18 - Uniform Controlled Substances Acto of 1992 Effective 7-1-2009Brian HarrisNo ratings yet
- Colorado Revised Statutes (CRS 12 36) Title 12 Article 36 and Article 36.5 Professions and Occupations, Medical Practice, Professional Review of Health Care ProvidersDocument57 pagesColorado Revised Statutes (CRS 12 36) Title 12 Article 36 and Article 36.5 Professions and Occupations, Medical Practice, Professional Review of Health Care ProvidersBrian HarrisNo ratings yet
- Colorado CRS 18 18 - Uniform Controlled Substances Acto of 1992 Effective 7-1-2009Document69 pagesColorado CRS 18 18 - Uniform Controlled Substances Acto of 1992 Effective 7-1-2009Brian HarrisNo ratings yet
- Colorado Revised Statutes (CRS 12 12)Document65 pagesColorado Revised Statutes (CRS 12 12)Brian HarrisNo ratings yet
- Colorado Guidelines of Professional Practice For Controlled Substances AddendumDocument14 pagesColorado Guidelines of Professional Practice For Controlled Substances AddendumBrian HarrisNo ratings yet
- Colorado Medical Board Policy Guidelines For The Use of Controlled Substances For The Treatment of Pain 12-36-117, C.R.S.Document5 pagesColorado Medical Board Policy Guidelines For The Use of Controlled Substances For The Treatment of Pain 12-36-117, C.R.S.Brian HarrisNo ratings yet
- Colorado Preferred Drug List (PDL) 2013Document25 pagesColorado Preferred Drug List (PDL) 2013Brian HarrisNo ratings yet
- Colorado Drug Control UpdateDocument7 pagesColorado Drug Control UpdateBrian HarrisNo ratings yet
- Colorado Guidelines of Professional Practice For Controlled SubstancesDocument65 pagesColorado Guidelines of Professional Practice For Controlled SubstancesBrian HarrisNo ratings yet
- Medical Injuries or Illnesses To Use Medical Marijuana in ColoradoDocument5 pagesMedical Injuries or Illnesses To Use Medical Marijuana in ColoradoGreenpoint Insurance ColoradoNo ratings yet
- Colorado Guidelines of Professional Practice For Controlled Substances AddendumDocument14 pagesColorado Guidelines of Professional Practice For Controlled Substances AddendumBrian HarrisNo ratings yet
- Colorado Guidelines of Professional Practice For Controlled SubstancesDocument65 pagesColorado Guidelines of Professional Practice For Controlled SubstancesBrian HarrisNo ratings yet
- Colorado House Bill 11-1043 Concerning Medical Marijuana, and Making An Appropriation ThereforDocument24 pagesColorado House Bill 11-1043 Concerning Medical Marijuana, and Making An Appropriation ThereforBrian HarrisNo ratings yet
- Colorado Licensing of Controlled Substances Act 2013 Sunset ReviewDocument31 pagesColorado Licensing of Controlled Substances Act 2013 Sunset ReviewBrian HarrisNo ratings yet
- Colorado Medical Board Policy Guidelines Pertaining To The Release and Retention of Medical Records Policy Number 40-07Document2 pagesColorado Medical Board Policy Guidelines Pertaining To The Release and Retention of Medical Records Policy Number 40-07Brian HarrisNo ratings yet
- Colorado Medical Marijuana Laws, Statutes, and CodesDocument60 pagesColorado Medical Marijuana Laws, Statutes, and CodesGreenpoint Insurance ColoradoNo ratings yet
- 42 CFR Part 8 Opioid Drugs in Maintenance and Detoxification Treatment of Opiate Addiction Final RuleDocument28 pages42 CFR Part 8 Opioid Drugs in Maintenance and Detoxification Treatment of Opiate Addiction Final RuleBrian HarrisNo ratings yet
- ASAM The Role of The Physician in "Medical" MarijuanaDocument60 pagesASAM The Role of The Physician in "Medical" MarijuanaBrian HarrisNo ratings yet
- Colorado Guidelines of Professional Practice For Controlled SubstancesDocument65 pagesColorado Guidelines of Professional Practice For Controlled SubstancesBrian HarrisNo ratings yet
- Colorado Revised Statutes (CRS 12 42.5) Title 12 Article 42.5 Professions and Occupations Pharmacists, Pharmacy Businesses, and PharmaceuticalsDocument48 pagesColorado Revised Statutes (CRS 12 42.5) Title 12 Article 42.5 Professions and Occupations Pharmacists, Pharmacy Businesses, and PharmaceuticalsBrian HarrisNo ratings yet
- The DEA Position On MarijuanaDocument63 pagesThe DEA Position On MarijuanaBrian HarrisNo ratings yet
- 42 CFR Part 8 Opioid Drugs in Maintenance and Detoxification Treatment of Opiate Addiction Final RuleDocument28 pages42 CFR Part 8 Opioid Drugs in Maintenance and Detoxification Treatment of Opiate Addiction Final RuleBrian HarrisNo ratings yet
- Alcohol and Drug Abuse Division (ADAD) Substance Use Disorder Treatment Rules Colorado DHSDocument80 pagesAlcohol and Drug Abuse Division (ADAD) Substance Use Disorder Treatment Rules Colorado DHSBrian HarrisNo ratings yet
- Medical Professionals Authorized To Prescribe Medication Under Colorado LawDocument2 pagesMedical Professionals Authorized To Prescribe Medication Under Colorado LawBrian HarrisNo ratings yet
- AdfgDocument9 pagesAdfgicha shafiraNo ratings yet
- 920 FullDocument17 pages920 FullHeru SigitNo ratings yet
- Fat Loss ForewordDocument3 pagesFat Loss Forewordbookw0rmaNo ratings yet
- Water Safety Plans Rural Water Supply IndiaDocument32 pagesWater Safety Plans Rural Water Supply Indiapraveen kumarNo ratings yet
- CHN Study GuideDocument5 pagesCHN Study GuideAshNo ratings yet
- JobDescriptionDocument17 pagesJobDescriptionAnonymous YqCxXQ6wF5No ratings yet
- Household 1Document2 pagesHousehold 1ainun rapikaNo ratings yet
- Vis RabiesDocument2 pagesVis RabiesSanjay KumarNo ratings yet
- Employee Benefits Program by Etiqa Life Insurance BerhadDocument23 pagesEmployee Benefits Program by Etiqa Life Insurance BerhadNurain SarinaNo ratings yet
- 2023 AAN Myositis Autoantibodies FINALDocument53 pages2023 AAN Myositis Autoantibodies FINALEvelina ȘabanovNo ratings yet
- FOOTBALL (SOCCER) INJURY REPORTING FORM English WordsDocument3 pagesFOOTBALL (SOCCER) INJURY REPORTING FORM English WordsNinNo ratings yet
- Diamondfreezemel32r E82eenDocument11 pagesDiamondfreezemel32r E82eenGILI RELIABILITYNo ratings yet
- Brigada ZumbaDocument2 pagesBrigada ZumbaRommel DaysonNo ratings yet
- Practical Exercise 3 Pareto Diagram: DR Yousef Amer - School of Engineering University of South Australia Page 1 of 3Document6 pagesPractical Exercise 3 Pareto Diagram: DR Yousef Amer - School of Engineering University of South Australia Page 1 of 3HarisNo ratings yet
- PodStick ManualDocument40 pagesPodStick ManualMusafir QolbuNo ratings yet
- Lec4 د.زينDocument8 pagesLec4 د.زينMohammed JaberNo ratings yet
- Pulpal Emergency SeminarDocument44 pagesPulpal Emergency SeminarHoda ZainNo ratings yet
- The Indian History of Prenatal EducationDocument10 pagesThe Indian History of Prenatal Educationvaibhav gaikwadNo ratings yet
- Organizational Design Name of The Company and Organizational DesignDocument6 pagesOrganizational Design Name of The Company and Organizational Designtina g morfiNo ratings yet
- Septico Tank TreatmentDocument3 pagesSeptico Tank TreatmentfernandaNo ratings yet