Professional Documents
Culture Documents
Cerebrospinal Fluid
Uploaded by
Marie SalomonováCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Cerebrospinal Fluid
Uploaded by
Marie SalomonováCopyright:
Available Formats
Marie Salomonov; 4163930
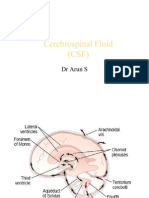
CEREBROSPINAL FLUID The cerebrospinal fluid (CSF) is a clear, colourless liquid which surrounds the spinal cord and brain. It has an important role in the immunological protection, metabolism and homeostasis of the CNS, as it provides a chemically stable environment, distributes nutritive materials and removes waste products; it also protects the brain from physical shocks and supports the venous sinuses (Agamanolis; 2013). CSF is found in the ventricular system inside the brain, major cisterns of the brain (e.g. cisterna magna, mesencephalic cistern), central canal of the spinal cord and in the subarchnoid space. In an adult the volume of CSF is between 140 to 270 ml and the rate of its production ranges between 0.2 to 0.7 ml per minute (Agamanolis; 2013). Apart from its function, this essay will describe CSF secretion and distribution and it will also briefly discuss some of its possible abnormalities and what these may indicate, emphasising the diagnostic use of CSF analysis. Production, Secretion and Absorption of the CSF The whole process of CSF production and secretion is achieved by combined processes of active transfer, diffusion and pinocytosis from arterial blood. Capillary filtration and epithelial secretory mechanisms are in place in order to control the chemical stability of the fluid (Kadel et al.; 2013).
Figure 1
Schematic diagram of CSF production and interfaces between blood, brain and CSF
Arrows indicate the passage of CSF from its formation at the choroid plexus to its absorption at the arachnoid villi (Terlizz et al.; 2006)
Marie Salomonov; 4163930
Some of the CSF is formed along ventricular walls and blood vessels and flows through perivascular channels, however, most of the CSF is produced in the lateral and fourth ventricles by a network of capillaries with fenestrated endothelial cells called choroid plexuses. These capillaries are covered by smooth ependymal cells, a type of As blood plasma enters the neuroglial cells with bulbous microvilli, which are linked by tight junctions that prevent the blood plasma from directly entering the ventricles. ependymal cells it is filtered while passing into the ventricles, forming CSF. The ependymal cells are thus said to control the composition of the CSF and maintain a blood-CSF barrier (Agamanolis; 2013). The circulation of CSF is propelled by the movement of cilia of ependymal cells as well as by the pulsation of the choroid plexus. From the lateral ventricles where most of the CSF is produced, the CSF passes through the foramen of Monro into the third and then through the cerebral aqueduct into the fourth ventricle. Most of the CSF then passes through a space within the meninges of the brain, and the the two subarachnoid space, through the medial foramen of Magendie lateral
Figure 2 Cerebrospinal fluid dynamics
foramina
of
Luschka
(Gray;
1918). Until it is reabsorbed into the venous circulation by the arachnoid granulations, the CSF then circulates
Arrows indicate the circulation of CSF (Brian et al.; 2002)
over the cortical surface of the brain and down the spinal canal. The CSF flowing over the cortical surface extends into the sulci in extensions of the subarachnoid space along blood vessels (Virchow-Robin spaces). In these perivascular spaces small solutes freely diffuse between the CSF and interstitial fluid and through the ependymal lining of the ventricular system. This process serves the movement of brain metabolites and thus helps to maintain the chemically stable environment of the CNS. Interstitial fluid flow across the ependymal surface into the ventricular system is also thought to be the source of a small amount of CSF (extrachoroidal CSF production) (Kandel et al.; 2013).
2
Marie Salomonov; 4163930
CSF leaves the cerebrospinal spaces in various locations and this process is often described as a dual as the fluid is reabsorbed to the venous circulation as well as drained into lymphatic vessels by way of perineural course. The return of CSF to the blood is managed through arachnoid granulations (clusters of villi) and villi which are located in the dural sinuses of the meninges and act as one-way valves between these sinuses and the subarachnoid space, whereas the absorption of CSF into the lymphatic system takes place around the cranial and spinal canal. It was established that the greater the CSF pressure is, the higher the rate of its absorption, suggesting that the rate of CSF absorption correlates with the CSF pressure (Gray; 1918). CSF Pressure Normal CSF pressure measured by a lumbar puncture is 8-15mmHg when the patient is lying on side lateral decubitus position, or 16-24mmHg for a sitting patient. It is usually assumed that the pressures are equal throughout the neuraxis when the patient is lying down, thus the CSF lumbar pressure can be used to estimate intracranial pressure. The Monro-Kellie doctrine states that an increase in volume of either brain tissue, blood, CSF, or other brain fluids will increase intracranial pressure, as the bone rigidity fixes the total cranial volume. Arterial and intracranial venous pressure changes can also affect intracranial pressure (influencing intracranial blood volume and CSF dynamics) (Kandel et al.; 2013). Several pathological conditions are characterised by an increased intracranial pressure, including brain oedema (increase in brain volume because of increased water content), or hydrocephalus (increase in volume of the cerebral ventricles) (Agamanolis; 2013). Normal CSF Composition and Pathological Abnormalities Under normal physiological conditions the CSF is clear in colour and together with the extracellular fluid of the brain it is in steady state and in osmotic equilibrium with the blood plasma. Compared to blood plasma, CSF is more acidic and has lower concentrations of K+, Ca2+, bicarbonate and glucose (Kandel et al.; 2013). In the table below, the average values of the major blood plasma and CSF constituents are compared.
Marie Salomonov; 4163930
Table 1 Comparison of Blood Plasma and Cerebrospinal Fluid Composition CSF* Water (%) Protein (mg/dL) Glucose (mg/dL) Osmolarity (mOsm/L) Na+ (mEq/L) K+ (mEq/L) Ca2+ (mEq/L) Mg2+ (mEq/L) Cl- (mEq/L) pH 99 35 60 295 138 2.8 2.1 0.3 119 7.33 Blood Plasma* 93 7000 90 295 138 4.5 4.8 1.7 102 7.41
*Average or representative values (Kandel et al.; 2013)
CSF analysis is a very useful tool when diagnosing diseases. variety Sample of of CSF neurological is usually
obtained by a and cell
lumbar puncture and More sophisticated
analysed for levels of protein and glucose count. methods (e.g. detection of oligoclonal bands) can be used to diagnose an ongoing inflammatory condition, such as multiple sclerosis. CSF of patients with multiple sclerosis also often contains myelin protein fragments and myelin 2013). basic Recent (Agamanolis;
Figure 3 Lumbar Puncture
studies showed that presence of three
The procedure involves insertion of a needle between the third and fourth lumbar vertebrae in the back and extracting a fluid sample. (2007 Terese Winslow)
specific protein biomarkers (amyloid 1-42, tau protein and P-Tau181P) in the CFS can indicate Alzheimers disease (Meyer et al.; 2010). Low glucose levels in CFS are often caused by leukocytes and/or tumour cells presence, as these consume glucose. Low glucose levels in the CSF may thus indirectly indicate suppurative, tuberculous and fungal infections or sarcoidosis as well as meningeal dissemination of tumours (Ravel; 1995). If blood is found in the CFS, it can indicate a subarachnoid haemorrhage and neutrophils and mononuclear cells might be indicative of meningeal irritation. Following
4
Marie Salomonov; 4163930
subarachnoid haemorrhage, the CFS colour changes to blonde (xanthochromia). This is caused by oxyhemoglobin and bilirubin. Sometimes this faint yellow, or even brown colouration can be observed by haemorrhagic infarcts, jaundice, and brain tumours. In patients with brain tumours (e.g. medulloblastoma), tumour cells may be found in the CSF. This usually indicates dissemination of the tumour in the subarachnoid space. A mononuclear inflammatory reaction is also one of the markers of brain tumours which can be found in the CSF (Ravel; 1995). Pleocytosis (increased inflammatory cells) may indicate infectious as well as noninfectious processes. Acute suppurative meningitis is indicated by polymorphonuclear pleocytosis, whereas mononuclear cells can be seen in viral infections, such as meningoencephalitis or aseptic meningitis, as well as tuberculous meningitis, multiple sclerosis, syphilis and neuroborreliosis (Agamanolis; 2013). Severe increase in CSF protein can be seen in individuals with Guillain-Barr syndrome and protein in CSF may also rise up to 500 mg/dL in patients suffering from bacterial meningitis. In inflammatory diseases of meninges (meningitis, encephalitis) the increase of protein is more moderate. Moderate protein increase can be also observed in patients suffering from subarachnoid haemorrhage, intracranial tumours, and cerebral infarction (Seehusen et al.; 2003). Challenges of Drugs Targeting the CNS Finally, after the brief description of possible pathologies which can be identified from patients CSF samples, the challenges of treatments of the different CNS pathologies should not be omitted in the conclusion. As described at the beginning of this essay, the CSF has an important role in immunological protection and maintenance of a chemically stable environment of the CNS which is distinct from the rest of the bodys environment. The tight junctions between the cerebral endothelial cells, between choroid plexus epithelial cells and at the blood-brain interface constitute the underlying control mechanism of the brains internal environment. This mechanism is based on diffusion restriction (tight junctions in the blood-brain and blood-CSF barriers) and different transport mechanisms which allow transport of allowed molecules (electrolytes, glucose, vitamins, peptides and amino acids) in and out of the brain and CSF (Saunders et al.; 1999). Analysis of a sample of the CSF allows for diagnosis of various neurological diseases, as the CSF composition can reveal certain biological markers specific to different diseases. If the delicate environment of the CNS is affected by an infection, or exhibits other
5
Marie Salomonov; 4163930
pathological symptoms, the drugs used to treat these must have specific physical and chemical properties in order to penetrate the above described barriers and get to their site of action. The barriers protecting CNS present a significant limiting factor as far as
pharmacological treatments of CNS diseases are concerned. It is assumed that lipid solubility and molecular size are one of the key factors which determine whether or not the drug can enter the CNS (Saunders et al.; 1999), however identification of chemical features which would allow entry of drugs into the brain and are important for the barriers permeability remain to be of major interest in the development of new CNStargeting drugs.
Marie Salomonov; 4163930
References Agamanolis; Neuropathology An illustrated interactive course for medical students and residents; Neuropathology-web.org, 2013. http://neuropathologyweb.org/chapter14/chapter14CSF.html. [Accessed on 13/4/2013]. Brian et al.; Atlas of Anaesthesia, Volume 2: Chapter 7; Current Medicine, 1998; Springerimages.com, 2002. http://www.springerimages.com.ezproxy.nottingham.ac.uk/Images/MedicineAndPublic Health/2-ACA0201-07-026. [Accessed on 15/4/2013]. Gray; Anatomy of the Human Body; Lea & Febiger, 1918; Bartleby.com, 2000. www.bartleby.com/107/. [Accessed on 13/4/2013]. Kandel et al.; Principles of Neural Science, Fifth Edition; McGraw-Hill Companies, Inc., 2013. Meyer et al.; Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people; Archives of Neurology, 2010; 67(8):949-956. Ravel; Clinical Laboratory Medicine: Clinical Application of Laboratory Data; Mosby, Inc., 1995. Saunders et al.; Barrier Mechanisms in the Brain, I. Adult Brain; Clinical and Experimental Pharmacology and Physiology, 1999; 26(1):11-19. Seehusen et al.; Cerebrospinal Fluid Analysis; American Family Physician, 2003; 68(6):1103-1108. Terlizz et al.; The function, composition and analysis of cerebrospinal fluid in companion animals: Part I Function and composition; The Veterinary Journal, 2006; 172(3):422-431.
You might also like
- Cerebrospinal FluidDocument31 pagesCerebrospinal FluiddrarunraoNo ratings yet
- Cerebrospinal FluidDocument18 pagesCerebrospinal Fluidmiss.JEJE100% (1)
- Anatomy and Physiology of Cerebrospinal Fluid 2011Document8 pagesAnatomy and Physiology of Cerebrospinal Fluid 2011Camilo Benavides BurbanoNo ratings yet
- Cerebrospinal, Pleural and Ascitic Uids: Case 1Document6 pagesCerebrospinal, Pleural and Ascitic Uids: Case 1Amelia PebriantiNo ratings yet
- The Normal CSFDocument2 pagesThe Normal CSFUm MahmoudNo ratings yet
- Cerebrospinal Liquor and Its CirculationDocument13 pagesCerebrospinal Liquor and Its CirculationusergrNo ratings yet
- Cerebrospinal Fluid: Dr. Usman Farooq (PT) Lecturer NWIHS DPT, MS-NMPTDocument14 pagesCerebrospinal Fluid: Dr. Usman Farooq (PT) Lecturer NWIHS DPT, MS-NMPTtwity 1No ratings yet
- Cerebrospinal FluidDocument154 pagesCerebrospinal Fluidabhirox69100% (1)
- Cerebrospinal FluidDocument29 pagesCerebrospinal FluidRiyan SaputraNo ratings yet
- Cerebrospinal Fluid: Physical Characteristic and Composition of The Cerebrospinal FluidDocument5 pagesCerebrospinal Fluid: Physical Characteristic and Composition of The Cerebrospinal FluiderickNo ratings yet
- PathologyDocument47 pagesPathologySubburaj Thiruvengadam100% (1)
- Apocalypse of AdamDocument11 pagesApocalypse of AdamTristan MarshallNo ratings yet
- CNS RosettesDocument5 pagesCNS RosettesSick DuckNo ratings yet
- Jurnal PDFDocument14 pagesJurnal PDFIcha IchaNo ratings yet
- DR Kaushal CSF Physiology PresentationDocument39 pagesDR Kaushal CSF Physiology Presentationoddie333No ratings yet
- Liquor CerebrospinalisDocument25 pagesLiquor CerebrospinalisIhda ParidahNo ratings yet
- CSFDocument40 pagesCSFDrNaveen Singh Rajpurohit KaduNo ratings yet
- Cyber PolygonDocument44 pagesCyber PolygonLeonardoNo ratings yet
- Classical Radiological SignsDocument2 pagesClassical Radiological SignsMuntaha JavedNo ratings yet
- Brain Imaging in Colloid CystDocument5 pagesBrain Imaging in Colloid CystMohamed Farouk El-FaresyNo ratings yet
- Embryology Respiratory System: Formation of Lung BudsDocument1 pageEmbryology Respiratory System: Formation of Lung Budsrezairfan221No ratings yet
- Brain Ventricles Al-Mubin 007Document25 pagesBrain Ventricles Al-Mubin 007Miazan SheikhNo ratings yet
- Molar Pregnancy PDFDocument8 pagesMolar Pregnancy PDFEgaSuharnoNo ratings yet
- CSF and Cerebral Blood FlowDocument58 pagesCSF and Cerebral Blood FlowCindy PrayogoNo ratings yet
- Principia DiscordiaDocument85 pagesPrincipia DiscordiaAbraham OgdenNo ratings yet
- Evolution of The Concept of Soul PDFDocument25 pagesEvolution of The Concept of Soul PDFLeandro BertoncelloNo ratings yet
- Fluid CerebrospinalDocument3 pagesFluid CerebrospinalAurelia AlexandraNo ratings yet
- OMS Central Nervous SystemDocument312 pagesOMS Central Nervous SystemDenisa Gherman100% (1)
- Infertile Couple - Role of Ultrasound FinalDocument31 pagesInfertile Couple - Role of Ultrasound FinalKalpavriksha1974No ratings yet
- Ἐξουσία - Exousia ShortDocument9 pagesἘξουσία - Exousia ShortDaniel ErgichoNo ratings yet
- Foldings of Embryo Foetal Period 1Document22 pagesFoldings of Embryo Foetal Period 1Riya SinghNo ratings yet
- Earwax and Foreign Body in Ear & NoseDocument29 pagesEarwax and Foreign Body in Ear & NosehashyNo ratings yet
- CSF Flowmetry MriDocument5 pagesCSF Flowmetry MribricklaneNo ratings yet
- Of Time and The MindDocument18 pagesOf Time and The MindAntonio LanotNo ratings yet
- 7) Realization of The Absolute (Jimmy de Bilde)Document18 pages7) Realization of The Absolute (Jimmy de Bilde)Jimmy De BildeNo ratings yet
- Edema CerebralDocument16 pagesEdema CerebralJose Ricardo MuñozNo ratings yet
- The Truth About The HyperwarDocument18 pagesThe Truth About The HyperwarKrisssszNo ratings yet
- in The Given Chest X-Ray, Normal Cardiothoracic Ratio IsDocument63 pagesin The Given Chest X-Ray, Normal Cardiothoracic Ratio Is李建明No ratings yet
- Part 4 Twelve Mind Powers Divine Wisdom Master Hilarion Charles Fillmore PDFDocument9 pagesPart 4 Twelve Mind Powers Divine Wisdom Master Hilarion Charles Fillmore PDFevans thitaiNo ratings yet
- Cerebrospinal CSFDocument31 pagesCerebrospinal CSFRashid MohamedNo ratings yet
- Practical Approach To The Use of Immunohistochemical Markers Used in NeuropathologyDocument119 pagesPractical Approach To The Use of Immunohistochemical Markers Used in NeuropathologyJames Davis100% (1)
- Man The Best and Most Perfect of Gods CreaturesDocument7 pagesMan The Best and Most Perfect of Gods Creaturesgiorgio802000No ratings yet
- Unit 5 Medical UltrasoundDocument6 pagesUnit 5 Medical Ultrasoundtwy113No ratings yet
- HidrosefalusDocument10 pagesHidrosefalusNelly AstikaNo ratings yet
- Hepatobiliary Disorders: Katrina Saludar Jimenez, R. NDocument42 pagesHepatobiliary Disorders: Katrina Saludar Jimenez, R. NreykatNo ratings yet
- Neurovascular SonographyDocument539 pagesNeurovascular SonographyAlexandra MoraesNo ratings yet
- Thomas Church - The History of The Great Indian War of 1675 and 1676 (Philip's War) (1854)Document404 pagesThomas Church - The History of The Great Indian War of 1675 and 1676 (Philip's War) (1854)chyoung100% (1)
- Lesson 7 - Nervous, Endocrine, Reproductive, Urinary SystemDocument37 pagesLesson 7 - Nervous, Endocrine, Reproductive, Urinary SystemAlejandro GuerreroNo ratings yet
- Ultrasound in Fibroids Part 2Document1 pageUltrasound in Fibroids Part 2Dr Pankaj TalwarNo ratings yet
- Cerebrospinal FluidDocument15 pagesCerebrospinal FluidAdriana SandovalNo ratings yet
- Pathway, Show The Major Pathway of CSF Flow. Beginning in The Lateral Ventricles, CSF FlowsDocument2 pagesPathway, Show The Major Pathway of CSF Flow. Beginning in The Lateral Ventricles, CSF FlowsMary Scarlette CenaNo ratings yet
- Cerebrospinal FluidDocument7 pagesCerebrospinal FluidjesusmsenatorNo ratings yet
- HydrocephalusDocument13 pagesHydrocephalusKyunaNo ratings yet
- 107327481702400102Document3 pages107327481702400102agriNo ratings yet
- Hydrocephalus PDFDocument17 pagesHydrocephalus PDFEdna López100% (1)
- Body Fluid Chapter 1Document76 pagesBody Fluid Chapter 1kanakalakshmimbsfNo ratings yet
- Ana & Physio HydrocephalusDocument4 pagesAna & Physio HydrocephalusJane VargasNo ratings yet
- Brain Edema and Disorders of CSF CirculationDocument68 pagesBrain Edema and Disorders of CSF CirculationDr. Yuvraj LahreNo ratings yet
- Cerebrospinal Fluid Circulation and HydrocephalusDocument12 pagesCerebrospinal Fluid Circulation and HydrocephalusI'Jaz Farritz MuhammadNo ratings yet
- Literature Review - Alzheimer's and Parkinson's Diseases As Protein Pathologies and Their Animal ModelsDocument18 pagesLiterature Review - Alzheimer's and Parkinson's Diseases As Protein Pathologies and Their Animal ModelsMarie SalomonováNo ratings yet
- Genetically Modified Animals in Neuroscience ResearchDocument9 pagesGenetically Modified Animals in Neuroscience ResearchMarie SalomonováNo ratings yet
- HPA Axis in MDDDocument6 pagesHPA Axis in MDDMarie SalomonováNo ratings yet
- Mirror Neurone SystemDocument9 pagesMirror Neurone SystemMarie SalomonováNo ratings yet
- Notice: Agency Information Collection Activities Proposals, Submissions, and ApprovalsDocument1 pageNotice: Agency Information Collection Activities Proposals, Submissions, and ApprovalsJustia.comNo ratings yet
- Plant Pathogens andDocument3 pagesPlant Pathogens andPrashant Waghrulkar100% (1)
- Alcohol Research Paper OutlineDocument6 pagesAlcohol Research Paper Outlineafmctmvem100% (1)
- Atrial Fibrillation PathophysiologyDocument2 pagesAtrial Fibrillation PathophysiologyanabbabarNo ratings yet
- Icd 10Document54 pagesIcd 10gexchaNo ratings yet
- Transient Tachypnea of The Newborn (TTN)Document6 pagesTransient Tachypnea of The Newborn (TTN)Wivan Havilian DjohanNo ratings yet
- Obstructed LabourDocument24 pagesObstructed LabourNatukunda Dianah50% (4)
- Efficacy of Doctorvox On Mutational FalsettoDocument8 pagesEfficacy of Doctorvox On Mutational FalsettoANA CRISTINA MENDEZ DIAZNo ratings yet
- Traditional Indian MedicineDocument3 pagesTraditional Indian MedicineShashvita RatraNo ratings yet
- Biopsy InterpretationDocument474 pagesBiopsy InterpretationSandoz100% (1)
- Lecture 3 Innate ImmunityDocument26 pagesLecture 3 Innate ImmunitytimcarasNo ratings yet
- Rufenal EmulgelDocument2 pagesRufenal EmulgelDr.2020No ratings yet
- MedicalerrorDocument38 pagesMedicalerrorchanda jabeenNo ratings yet
- English: Quarter 4 - Module 7 Making GeneralizationsDocument16 pagesEnglish: Quarter 4 - Module 7 Making Generalizationslenra esoj lasorNo ratings yet
- Alpha Thalassemia PDFDocument21 pagesAlpha Thalassemia PDFAnonymous Yo0mStNo ratings yet
- P.E 3A Learning Activity Sheet WEEK 5 6Document7 pagesP.E 3A Learning Activity Sheet WEEK 5 6Ris CorreaNo ratings yet
- AminoglycosidesDocument20 pagesAminoglycosidesHassan.shehri100% (5)
- Test Name Result Unit Reference Interval EndocrinolgyDocument1 pageTest Name Result Unit Reference Interval Endocrinolgygeetha kodaliNo ratings yet
- Cosmetology Library ResourcesDocument40 pagesCosmetology Library Resourcesemiliana magerusanNo ratings yet
- VeneerDocument48 pagesVeneerAli IhsanNo ratings yet
- Dystolic Dysfunction Ppt. SalmanDocument42 pagesDystolic Dysfunction Ppt. SalmanMustajab MujtabaNo ratings yet
- 1 Title and SynopsisDocument11 pages1 Title and SynopsisMuhammad Arief ArdiansyahNo ratings yet
- Myths PDFDocument8 pagesMyths PDFLuisa Elena HernandezNo ratings yet
- Presentation 1Document12 pagesPresentation 1Rizka FarahinNo ratings yet
- SPM Biology 2019 State Trial Papers Item AnalysisDocument12 pagesSPM Biology 2019 State Trial Papers Item AnalysisNuan Ting NgNo ratings yet
- STS CRFDocument38 pagesSTS CRFYosoy LomasNo ratings yet
- 4UFZ75PF0FYRH8 4UFZ75PF0FYRH8 : Division of Forensic SciencesDocument7 pages4UFZ75PF0FYRH8 4UFZ75PF0FYRH8 : Division of Forensic SciencesAttila KissNo ratings yet
- The Endocrine System and Feedback MechanismsDocument8 pagesThe Endocrine System and Feedback MechanismsJame SmithNo ratings yet
- Rickey Dixon Letter To The Court On NFL Concussion Settlement FeesDocument9 pagesRickey Dixon Letter To The Court On NFL Concussion Settlement FeesRobert Lee100% (1)
- Tony Gaddis Python BookDocument5 pagesTony Gaddis Python BookArslan AliNo ratings yet