Professional Documents
Culture Documents
Fisiopato NCPH
Uploaded by
Juan Camilo MoralesOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Fisiopato NCPH
Uploaded by
Juan Camilo MoralesCopyright:
Available Formats
PATHOLOGY
RESEARCH AND PRACTICE
Urban & Fischer Verlag http://www.urbanfischer.de/journals/prp
Review
Pathology and Pathogenesis of Idiopathic Portal Hypertension with an Emphasis on the Liver
Yasuni Nakanuma1, Koichi Tsuneyama1, Makoto Ohbu2 and Kazuyoshi Katayanagi1
1
Department of Pathology (II), Kanazawa University School of Medicine, Kanazawa, and 2 Department of Pathology, Kitasato University School of Medicine, Sagamihara, Japan
Summary
Idiopathic portal hypertension (IPH) is characterized by a long-standing presinusoidal portal hypertension of unknown etiology in adults. Some unidentified agent(s) affect(s) the intrahepatic small portal veins or portal tracts. Immunological disturbance, thromboembolism, infectious etiology and/or increased fibrogenesis in portal tracts are suspected as being candidates for the primary agent(s). During the long clinical course of IPH, several pathological changes may occur, including subcapsular parenchymal atrophy, atrophy of the liver, portal and parenchymal fibrosis, and portal venous phlebosclerosis and thrombosis. The last-named of these lesions is mostly found in patients with a history of splenectomy. Subcapsular parenchymal and hepatic atrophy may result from a hepatocellular dropout via apoptosis or necrosis because of intrahepatic hemodynamic disturbances, particularly chronic portal venous blood insufficiency. Pericellular fibrosis and thin fibrous septa are also frequently found and associated with activated perisinusoidal cells positive for smooth muscle actin. At the same time, vague nodular hyperplasia of hepatocytes not surrounded by fibrous septa is not infrequently seen. It may resemble nodular regenerative hyperplasia, partial nodular transformation, or focal nodular hyperplasia. However, liver cirrhosis does not occur even at the terminal stage. Taking these findings into consideration, a new staging of IPH with a combination of hepatic parenchymal atrophy and portal venous thrombosis was proposed: non-atrophic liver without subcapsular parenchymal atrophy (stage I), non-atrophic liver with subcapsular parenchymal atrophy (stage II), atrophic liver with subcapsular parenchymal atrophy (stage III), and portal venous occlusive thrombosis (stage IV). IPH livers are likely to progress from stage I to stage III. Stage IV, which occurs relaPathol. Res. Pract. 197: 6576 (2001)
tively late, has a poor prognosis. This staging is applicable to clinical and autopsy cases without any histological data. Key words: Liver pathology Hepatic fibrosis Hepatocellular apoptosis Portal venous insufficiency Idiopathic portal hypertension
Introduction
Idiopathic portal hypertension (IPH) is characterized by a long-standing non-cirrhotic portal hypertension because of the intrahepatic block of small portal vein branches [32, 37]. Pathologically, portal phlebosclerosis, obliteration of intrahepatic small portal veins, and subcapsular parenchymal atrophy with unusual approximation of portal tracts and hepatic veins to each other are characteristic of IPH livers (Figs. 15) [25, 36]. Hepatocellular atrophy with sinusoidal dilatation and portal and intralobular fibrosis are also found in IPH livers, to various degrees and in various combinations [47]. Hepatocellular nodular hyperplasia is not uncommon, either [4, 25]. However, early or primary changes or lesions causally related to intrahepatic portal venous block have not yet been identified, although several etiological factors have been proposed. In this review, we first describe the clinical features and hemodynamic character of IPH. Then, we try to categorize the primary and secondary pathological
Address for correspondence: Yasuni Nakanuma, Second Department of Pathology, Kanazawa University School of Medicine, Kanazawa 920-8640, Japan. Tel.: ++88-076-265-2195, Fax: ++88-076-234-4229. E-mail: pbcpsc@kenroku.kanazawa-u.ac.jp
0344-0338/01/197/2-65 $15.00/0
66 Y. Nakanuma et al.
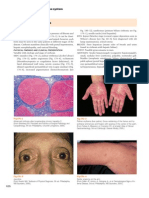
Fig. 1. Cut surface of autopsy liver of idiopathic portal hyperetnsion. The liver is atrophic, and portal tracts (large arrows) and hepatic veins (small arrow) are unusually approximated to the hepatic capsule. Intrahepatic large portal vein (V) is dilated.
Fig. 3. Small portal tract in idiopathic portal hypertension. This portal tract (arrow) is densely sclerotic, and hepatic artery and bile ducts are identifiable. However, portal vein is obliterated. HE. Fig. 4. Small portal tract in idiopathic portal hypertension. These portal tracts (arrows) are densely sclerotic because of marked elastosis. Portal vein is obliterated. Elastica van Gieson stain. Fig. 2. Histological section of autopsy liver of idiopathic portal hypertension. Portal tracts and hepatic vein tributaries are unusually close to the hepatic capsule, and the subcapsular hepatic parenchyma is atrophic. Arrow, hepatic capsule. HE. Fig. 5. A medium-sized portal tract in idiopathic portal hypertension. Portal tract is densely fibrotic, and a mediumsized portal vein (arrow) shows phlebosclerosis with muscular hyperplasia. HE.
Idiopathic Portal Hypertension 67
changes in IPH livers with respect to the progression of pathological changes as a consequence of long-standing hemodynamic alterations. The recent progress in the etiopathogenetic study of IPH is also reviewed. Finally, a new staging of IPH taking the progressive secondary hepatic lesions into consideration is proposed for a clearer and easier evaluation of the clinicopathological features of this disease. This review is based on 97 liver specimens from cases fulfilling the criteria for IPH obtained from the file of hepatobiliary diseases in our laboratory and affiliated hospitals.
A
Main Clinical Findings and Prognosis
Main clinical findings. IPH affects preferentially middle to old aged women in Japan, while the equivalent disease in India, non-cirrhotic portal fibrosis of the liver (NCPF), usually affects young men [24, 37]. This disease is characterized by overt splenomegaly with portal hypertension, and by relatively mild abnormalities in liver function tests [35]. There are no laboratory findings characteristic of IPH, and IPH patients have no stigmata of chronic liver disease, such as spider angiomas or palmar erythema. Levels of one or more blood elements are decreased, and pancytopenia is common. Serologically, IPH is not causally related to chronic infection of HBV or HCV. Radiologically, the established IPH livers show central hypertrophy and peripheral atrophy [20]. Esophageal varices and bleeding are also common. Prognosis of IPH. The disease is relatively benign if variceal bleeding is controlled or prevented, and does not progress to cirrhosis. However, some IPH cases with hepatic failure have been reported [5, 6, 14]. Ichimura et al. [14] followed 171 patients with IPH and reported that 20 patients died (6 from bleeding in the gastro-intestinal tract, 5 from hepatic insufficiency and 9 from other causes). Male patients with a disease onset at less than 40 years of age had poorer prognoses. The occurrence of occlusive portal venous thrombosis indicates poor prognosis, and in fact, in autopsy cases, occlusive portal venous thromboembolism is not infrequently encountered. In patients with collagen vascular disease [15, 45], an association with IPH is regarded as being one of the most important complications affecting prognosis.
Fig. 6. Intimal edema and fibrosis of portal vein branches: A small portal vein (arrows) shows intimal edema and fibrosis with mild lymphocytic infiltration (a kind of phlebitis). The outline of this vein is well delineated by Elastica van Gieson stain (B). V, hepatic arterial branch. A: HE; B: Elastica van Gieson stain. A and B are serial sections of the same portal tract.
Hemodynamics of IPH in comparison with liver cirrhosis
Portal vein pressure is significantly elevated in liver cirrhosis and IPH. In liver cirrhosis, portal venous blood flow into the liver is almost normal, while intrahepatic vascular resistance at the postsinusoidal level is
markedly increased [31]. In addition, hepatic arterial blood flow into the liver is increased, and there are many arterio-portal venous (AP) shunts in the liver. By contrast, in IPH there is an increase in the portal venous blood flow into the liver, in the diameter of portal vein trunks, and in presinusoidal vascular resistance, but a decrease in the hepatic arterial flow into the liver; A-P shunt is negligible. Extrahepatic portal-systemic shunts are present in IPH and liver cirrhosis. In cirrhosis, portography reveals distortion of the intrahepatic vasculatures and hepatofugal flow, and arteriograms show a characteristical corkscrew appearance. Although the portograms in IPH vary among cases, dilatation of the hilar portal veins and intrahepatic large branches, irregular and often obtuse-angled divisions of the peripheral branches, occasional abrupt interruptions, and an avascular area beneath the liver surface are frequently seen. Non-opacification of some of the large intrahepatic portal branches and a paucity of medium-
68 Y. Nakanuma et al.
sized portal branches could be interpreted as a late and secondary complication of portal venous thromboembolism [9]. Characteristic changes in IPH include frequent hepatic vein-to-hepatic vein anastomoses, narrower angles between large veins and their tributaries, smooth and wavy middle-sized to large branches (giving a general weeping willow appearance), homogeneous sinusoidal filling, and minimal to no filling of the portal venous system on wedged retrograde portography [10]. In cirrhosis, by contrast, changes include rare vein-to-vein anastomoses, wide angles between veins and tributaries, irregular stenosis of large veins and branches at various levels, spotty sinusoidal filling, and frequent retrograde flow in the portal venous system. IPH is also characterized by marked splenomegaly. Marked splenomegaly with increased blood inflow cannot be simply attributed to congestion, and it remains an enigma.
frequently expressed on the microvasculature of portal tracts in IPH; this expression may be a factor initiating the immunological assault on portal microvessels in IPH [45, 50, 51]. An increased level of interferon (IFN) in portal venous blood may be responsible for this HLA-DR expression [58]. As for the adhesion molecules, which are important in the interaction between lymphocytes and accessory and target cells, the serum level of soluble VCAM-1 is found to be increased [58]. The level of soluble ICAM1 of IPH patients is slightly elevated but not different from that in patients with other diseases. VCAM-1 is expressed in the sinusoidal lining cells and portal venous endothelial cells in several IPH patients. Increased expression of VCAM-1 may reflect an immunopathological phenomenon in the occurrence of IPH.
Progressive fibrosis
Etiopathogenesis of IPH
The primary factor(s) involved in the development of presinusoidal portal hypertension may vary with the etiopathogenesis. In this context, IPH could be heterogeneous. The following points may represent factors playing a role in the etiology of IPH.
Immunological factors
A number of immunological abnormalities, such as hypergammaglobulinemia and antinuclear antibodies with high titer, occur in patients with IPH. LE cell phenomena were also positive in some patients [15, 23]. In addition, IPH associated with systemic lupus erythematosus (SLE), chronic thyroiditis, mixed connective tissue disease, and progressive systemic sclerosis (PSS) has been reported [26, 28, 29, 50, 51, 42]. The presence of anticardiolipin antibody, indicated by positivity for lupus anticoagulant, is suggestive of the presence of a common immunological mechanism in the etiology of IPH and SLE. Raynauds phenomenon associated with positive anti-RNP antibody is also associated with IPH. Usually, IPH and other autoimmune or collagen vascular diseases develop simultaneously [45]. Immunopathologically, in IPH livers, there are lymphoid cells in portal tracts, particularly at early stages [32]. Surgical specimens show more lymphoid cells. As described below, edema and fibrosis with lymphocytic infiltration into the intima of the small portal veins is also known to occur in IPH livers (Figs. 6A, 6B) [29, 51]. These lymphoid cells may be involved in the immunopathological processes of IPH [58]. HLA-DR antigen, which is involved in immune recognition and other immunological reactions, is more
Connective tissue growth factor (CTGF) stimulates in vitro fibroblast proliferation and the synthesis of extracellular matrix [40]. It is known that the serum level of CTGF is increased in patients with PSS and pulmonary fibrosis. In addition, it appears that production of CTGF is involved in the development or maintenance rather than the initiation of fibrosis in PSS. Recently, increased serum levels of CTGF were found in IPH patients (Tsuneyama et al, in preparation). There are case reports of IPH with PSS, suggesting that CTGF is an important fibrogenetic factor in situ in portal venous fibrosis of IPH, as speculated in PSS. In the group with chronic hepatitis, an increase in CTGF immunostaining was associated with a higher score of fibrosis; CTGF was strongly expressed during liver fibrogenesis, and hepatic stellate cells seemed to be the major cellular source of CTGF in the liver [40]. In IPH, similar processes are effective, although the exact mechanism of the fibrogenesis in portal fibrosis remains speculative.
Thrombosis and clotting abnormalities
Mural and even occlusive thromboembolism of hilar and intrahepatic large portal veins occur secondary to IPH (Fig. 7), an event that may aggravate the liver pathology of IPH. In portal venous thrombosis, deficiencies in natural anticoagulants, such as protein C and factor V Leiden mutation, are strongly associated with thrombosis [7, 39, 54]. The deficiencies produce a favorable medium for thrombus generation in the portal vein [7]. In Japan, direct evidence for portal venous thrombosis as a primary factor of IPH is still lacking [35]. The calculated incidence of portal vein thrombosis was angiographically 0.573% of all cirrhotic patients without splenectomy in the past [37], although the incidence of portal vein thrombosis unrelated to splenectomy was angiographically 2.86% in IPH.
Idiopathic Portal Hypertension 69
cy, while some hepatic and portal changes may be related to the primary or initiating factor(s). Histological heterogeneity is a fundamental character of IPH. In advanced cases, enlarged portal tracts are distributed unevenly and often positioned closely together, so that the normal lobular or acinar architecture is profoundly distorted. Subcapsular parenchymal atrophy is evident [19, 37]. In other cases, the liver histology is almost normal, except for portal venous dilatation or obliteration.
Hepatic parenchyma and hepatic vein tributaries of IPH livers
Fig. 7. The cut surface of autopsy liver of idiopathic portal hypertension. Intrahepatic portal veins are occluded by organizing and fresh thromboemboli (large arrows). Portal tracts and hepatic veins (small arrows) are unusually approximate to the hepatic capsule.
The acinar architecture is distorted as follows: 1) formation of isolated aberrant vessels with a random distribution; 2) displaced and abnormally large hepatic vein branches and 3) slender, curved fibrous septa (hairline septa). The histological changes include the following lesions: Hepatic parenchymal atrophy. Atrophy of IPH livers is recognizable by three parameters. First, the weight of the liver is reduced in about one half of the cases, although there is a wide variation. The IPH liver is usually atrophic, especially at a late stage. Next, the atrophy of hepatic parenchyma is recognizable by atrophy of hepatic lobules (Fig. 8) [36], an observation preferentially made in perivenular areas and also between hyperplastic hepatocellular nodules; hepatocytes are more or less atrophic or small. Congestion is sometimes seen in these atrophic areas. Third, on gross examination atrophy tends to occur at the hepatic periphery or in subcapsular regions (subcapsular hepatic parenchymal atrophy) (Figs. 1, 2, 7). There is unusual approximation of portal tracts and hepatic vein tributaries close to the hepatic capsule. In these areas, hepatic parenchyma is variably lost.
Infectious etiology
That the number of IPH patients is decreasing in developed countries might be attributed to improvements in the environment or public health. To obtain clues as to the etiopathogenesis of IPH, an attempt was made to produce a hepatic lesion similar to that in IPH by repeated injection of aggregated killed non-pathogenic E. coli directly into the portal vein [18, 49]. In the treated dogs, the histology of the liver showed dense fibrosis in the portal tract and aberrant vasculatures around the portal area. Portal pressure was elevated and middle-tosmall-sized portal branches were decreased in number, as revealed by portography. These changes closely mimic those seen in human IPH. It is possible that the appearance of an antigen, such as bacteria from the intestine to the portal venous system, plays an etiologic role in IPH.
IPH related to toxic or chemical environmental factors
Presinusoidal portal hypertension resembling IPH is known to develop in patients treated with chemotherapeutic agents and during arsenic intoxication and vinyl chloride polymerization [13, 52]. The histological and clinical hemodynamic similarity of these cases to IPH suggests that IPH sometimes may result from unknown toxic, possible environmental, chemicals and drugs.
Pathology of IPH Livers
The pathological features of IPH livers are basically non-pathognomonic. Most of these changes could be the result of a long-standing portal venous insufficienFig. 8. Histology of idiopathic portal hypertension. Portal tracts and hepatic vein tributaries are crowded, suggesting hepatic atrophy and small sized hepatic lobules. Elastica van Gieson staining.
70 Y. Nakanuma et al.
Pathogenesis of hepatic parenchymal atrophy: Disordered intrahepatic hemodynamics, particularly chronic portal venous insufficiency, may be responsible for the progression of hepatic parenchymal atrophy [1, 36]. In experimental animals, ischemia, particularly portal venous insufficiency, is known to cause hepatocellular atrophy and apoptosis [1, 16, 22, 57]. Wanless et al. [47] also reported that atrophy and nodular hyperplasia constitute a chronic response to ischemia, and that vascular obliteration is an important cause of apoptosis and atrophy, which are found in chronic liver diseases including IPH. Hepatocellular apoptosis is a process of ischemic hepatic parenchymal atrophy or dropout. Two types of eosinophilic hepatocellular changes are presumed to be apoptotic in origin. This was defined by round acidophilic bodies and stellate-shaped acidophilic bodies [47]. Either of these apoptotic changes is usually found focally in the hepatic parenchyma in IPH. They are more or less frequent in areas with sinusoidal dilatation. Focal necrosis is characterized by the dropout of hepatocytes and lymphoid cell infiltration. Focal necrosis is occasionally associated with pigmented macrophages scattered throughout the hepatic parenchyma of IPH. Parenchymal collapse is probably due to portal venous occlusion, such as thromboembolism, which is also superimposed in IPH livers, particularly at terminal stages. Occasionally, the hepatic segment collapses totally. Waving of the hepatic surface is characterized by granularity of the hepatic capsule and loss of hepatic parenchyma. The hepatic surface is nodular, appearing as liver cirrhosis. This is an extreme feature of peripheral hepatic parenchymal atrophy with fibrous overgrowth. In the deeper areas, such fibrosis is minimal, and so is nodularity. Parenchymal fibrosis. Hepatic parenchymal fibrosis is divided into three categories: pericellular fibrosis, intralobular slender fibrous septa, and slender fibrous septa from the portal tracts. These changes are more or less mild and focal, when compared to other fibrotic progressive liver diseases. Pericellular fibrosis found around the hepatic cords is frequently seen in the perivenular areas, and also between hyperplastic hepatocellular nodules (Fig. 9). In these areas, hepatocytes are atrophic, and sinusoids were dilated, linking neighboring central veins. Intralobular fibrous slender septa were seen in more than 50% of IPH cases. Slender fibrous septa from the fibrotic portal tracts are seen focally in IPH. These fibrous septa link neighboring portal tracts. Pathogenesis of parenchymal fibrosis: Microcirculatory changes, particularly portal venous insufficiency, may be related to pericellular fibrosis. Interestingly, smooth muscle antigen (ASMA)-positive, activated perisinusoidal cells (myofibroblasts) are increased or
Fig. 9. Histology of idiopathic portal hypertension. A: In perivenular areas, hepatocytes are atrophic with condensation of perisinusoidal fibers (V). P, portal tract. Reticulin stain. B: In perivenular areas there is deposition of collagen fibers (arrow) around atrophic hepatocellular cords. HE.
accumulated in the perivenular areas and also around atrophic hepatocytes in the hepatic parenchyma (Fig. 10). Activated perisinusoidal cells are known to actively produce extracellular matrix proteins, including collagen, laminin and fibronectin [8,11,41,43,44,59]. Portal venous insufficiency may activate perisinusoidal cells and then contribute to the development and progression of mild fibrosis in IPH. Sinusoidal dilatation and aberrant blood vessels. Sinusoidal dilatation is not infrequent in IPH. In some cases, the sinusoidal dilatation is associated with fibrous septa and is related to aberrant blood vessels in the hepatic parenchyma. Aberrant blood vessels, which are defined as dilated blood vessels in the hepatic parenchyma immediately adjacent to the peripheral portal tract (Fig. 11), appear under conditions of extrahepatic portal obstruction and IPH [36]. Ohbu et al. [30] showed that aberrant vessels are frequently found in IPH and extrahepatic portal obstruction. Aberrant vessels demonstrate the same immunoreactivity as do portal veins for collagen type IV, laminin, factor VIII and ulex europaeus agglutinin-I. It has been concluded that they arise from the vasa septalis or inlet venules, which would be used as intrahepatic shunts draining portal blood flow blocked by stenosed portal veins. Increased portal pressure would be expected to enhance the development of aberrant vessels. Nodular hepatocellular hyperplasia. Nodular regeneration is found in one third or more of IPH cases (Fig. 12) [4, 25]. Atrophy and nodular hyperplasia of the hepatic parenchyma are regarded as constituting a chronic response to ischemia in IPH [47], although it remains unclear why hepatocellular hyperplasia is predominant in
Idiopathic Portal Hypertension 71
some cases and absent in others. These nodular changes are similar to nodular regenerative hyperplasia, focal nodular hyperplasia, partial nodular transformation and macroregenerative nodules of the liver, although this
changes are incomplete or immature in their morphologies and/or focal in their distribution in IPH, as compared to those without portal hypertension . Hepatic vein tributaries and terminal hepatic venules. Some terminal and sublobular hepatic veins show phlebitis or some eccentric fibrosis, without clear evidence of luminal compression or occlusion [36]. Small hepatic veins also frequently show fibrous thickening of their walls.
Portal fibrosis and vascular damage
10
Changes in the portal tract include fibro-elastosis, phlebosclerosis, portal venous dilatation and obliteration, and capillary dilatation. Pathological changes of portal tracts differ depending on the size of the portal tracts; these are also quite heterogeneous and dependent on the place and the patient, probably reflecting the stage or progression of IPH. Portal fibrosis and periductal fibrosis. Portal tracts show dense collagen deposition. They are variably enlarged, although they are usually round and occasionally show spike-like fibrous septa growing into the hepatic parenchyma. A lot of elastic fibers are also present. Disordered synthesis and degradation of fibrosis and extracellular matrix (ECM) may be responsible for dense portal fibrosis. Portal tract inflammation characterized by infiltration of lymphoid cells is also seen, particularly in wedge biopsy cases. Periductal fibrosis is also frequently seen at the level of medium-sized interlobular bile ducts and septal bile ducts. This may be due to an insufficiency of peribiliary vascular plexus, because their changes resemble the adverse effects of transcatheter arterial embolization therapy or chemoembolization [17]. It is possible that portal venous vascular compression by portal fibrosis or arterial hypoperfusion leads to the ductal changes in IPH. Loss of bile ducts is a rare complication [27]. Vascular changes Grossly visible intrahepatic portal veins including hilar portal veins and extrahepatic portal veins These are open or even dilated in established IPH livers, although they constantly show phlebosclerosis and intimal thickening to various degrees and distribution, suggesting the organization of repeated mural and possibly occlusive thromboembolism. The medial muscle is also increased (arterialization). Fresh occlusive thromboembolism, which is often superimposed, is frequent in autopsy cases. Thrombotic processes of the portal venous system eventually occur, particularly after splenectomy [50]. Portal venous blood stasis may be responsible for this. Some main branches of the portal vein are occasionally occluded by an old thrombus
11
12
Fig. 10. Immunostaining of -smooth muscle antigen (SMA) in idiopathic portal hypertension. Perisinusoidal cells in perivenular areas are positive for -SMA and increased in the perivenular area. V, central vein; P, portal tract. Fig. 11. Histology of idiopathic portal hypertension. Arrows denote aberrant vessels in the hepatic parenchyma adjacent to fibrotic portal tract (P) in which original portal veins are obliterated. HE. Fig. 12. Histology of idiopathic portal hypertension. Vague nodular hyperplasia of hepatocytes is seen (N) in the hepatic lobule. P, portal tract. Reticulin stain.
72 Y. Nakanuma et al.
with recanalization, and extrahepatic portal venous obstruction of adults with features of IPH in the liver can be included in IPH (Fig. 7) [38]. Small portal vein branches Portal venous obliteration or luminal narrowing is a rather constant finding, particularly at autopsy [33], although portal veins are dilated and some have herniated into the surrounding hepatic parenchyma, particularly in needle biopsy specimens. Phlebosclerosis of medium-sized intrahepatic portal veins is a frequent and constant lesion that is recognizable at wedge biopsy or autopsy. This lesion is associated with muscular hypertrophy. Medium-sized portal vein branches occasionally show intimal thickening and even recanalization. In addition, edematous and fibrous thickening of the intima with inflammatory cell infiltration in the intima of portal vein branches is occasionally seen (Figs. 6A, 6B). This lesion may be a kind of phlebitis. Although this lesion is focal, it is rather characteristic of IPH and may be a primary lesion of IPH according to Ohbu [29]. It is exclusively found in IPH livers, which may reflect the immunological pathogenesis of IPH. Lymphatics. The number of lymphatics was higher in the IPH samples than in the control samples, suggesting that the increased lymph flow may in turn reduce the high portal vein pressure in IPH. Hepatic arterial changes. Our preliminary study disclosed that hepatic arterial branches are increased neither in number nor in the luminal area in IPH, reflecting the decreased arterial blood flow in the liver.
Diagnosis of IPH by needle or wedge biopsy
under different names, such as hepatoportal sclerosis, NCPF, non-cirrhotic intrahepatic portal hypertension, and benign intrahepatic portal hypertension [1, 22]. They share not only clinical and laboratory features but also pathological features, such as portal venous and portal tract abnormalities, and also parenchymal atrophy perhaps as a result of portal circulatory insufficiency [36]. The clinical and pathological features of these diseases may be the same, although their etiopathogenesis may differ according to location. In addition, extrahepatic portal venous obstruction with clinicopathological features of IPH could be included in the category of IPH. Such cases must be IPH originally, because there are several IPH cases in which portal venous thrombosis and portal venous obstruction occur secondarily in the course of IPH (stage IV, see below). In addition, there are also patients with spontaneous portal-systemic shunt and hepatic encephalopathy without splenomegaly. The liver histology of these patients resembles IPH but with normal portal pressure [48]. Such cases could also constitute a variant of IPH in which portal blood flow into the shunt and thereby clinically detectable portal hypertension and splenomegaly do not occur. Related diseases. Incomplete septal cirrhosis: In incomplete septal cirrhosis, slender septal fibrosis is an extension of portal fibrosis and perivenular fibrosis, and subdivides the parenchyma into inconspicuous nodules. These livers also show an abnormal spacing between the portal tracts and veins, and small hypoplastic portal tracts within the parenchyma. This could be a late manifestation of IPH[5, 6, 25], although the differentiation from macronodular cirrhosis is controversial in several cases. Nodular regenerative hyperplasia (NRH): This disease is characterized by non-cirrhotic regenerative nodules in the liver and occurs in portal hypertensive cases as well as in cases without portal hypertension. These nodulations generally involve the whole liver. Distinguishing NRH with portal hypertension from IPH with parenchymal nodular hyperplasia is difficult, and such cases could be included in IPH. Partial nodular transformation: This disease is characterized by grossly visible nodulations without fibrous septa, mainly involving the hepatic hilar regions. Some of these cases may occur as a compensatory hypertrophy of the liver in non-portal hypertensive cases, such as partial biliary occlusion, although the cases associated with non-cirrhotic portal hypertension may belong to IPH. Similar but different diseases. Myeloproliferative disorders: In myeloproliferative disorders such as myelofibrosis or polycythemia vera, non-cirrhotic portal hypertension can develop. In such cases, thrombosis of portal veins and hepatic veins is a common cause of
IPH is a clinical syndrome of pre-sinusoidal long-standing portal hypertension. Although the degree and distribution of the pathological changes related to IPH are generally heterogeneous, thus creating sampling errors for biopsy, histopathological study is necessary to render a diagnosis of IPH. The most important task for pathologists is to exclude liver cirrhosis, particularly macronodular liver cirrhosis. Biopsy specimens, particularly needle biopsy, not infrequently show nonspecific changes, i.e., non-diagnostic changes. Liver biopsies reveal portal fibrosis with subintimal thickening or luminal obliteration of terminal portal vein branches, and a striking peri-ductal fibrosis, supporting the diagnosis of IPH [53]. When portal hypertension occurs in association with a radiologically patent portal vein and an essentially normal liver biopsy specimen, IPH must be considered.
Comparison with equivalent and related diseases
Equivalent or similar diseases. Diseases similar to IPH occur worldwide. These, however, are known
Idiopathic Portal Hypertension 73
portal hypertension [56]. However, in a few cases with portal hypertension, increased portal blood flow from marked splenomegaly associated with extramedullary hematopoiesis could play a role in portal hypertension. Nodular regenerative hyperplasia of the liver is also encountered [55]. Presinusoidal portal hypertension related to medical treatments and intoxication: There is an association between a long-standing presinusoidal portal hypertension resembling IPH and the use of chemotherapeutic agents, particularly thioguanine [46]. Mild sclerosis of some small portal triads and perisinusoidal fibrosis are the only abnormalities seen. Chronic arsenic intoxication, usually for psoriasis, is also known to be associated with such a syndrome [13]. A peculiar pattern of progressive portal tract, inconspicuous intralobular and conspicuous capsular fibrosis accompanied by splenomegaly was also observed in five workers with vinyl chloride polymerization [52]. Hypertrophy and hyperplasia of both hepatocytes and hepatic and splenic mesenchymal cells were also seen [61]. Furthermore, a similar syndrome occurs following renal transplantation. These patients had been treated with azathioprine and prednisolone for several years. In these patients, the spleen might have played an important role in the development of this syndrome. Microscopic examination of liver biopsies taken at the operation revealed lymphoplasmacytic infiltration with bile duct hyperplasia but no evidence of periportal fibrosis except for very mild perisinusoidal fibrosis.
sy specimens. The parenchymal nodular changes of the liver are not recommended to be used for the staging of IPH livers either, because they are not always present, and the correlation between them and the progression of IPH remains unclarified. Therefore, we adopted three main, grossly recognizable, morphological factors in this new staging, i.e., the presence or absence of peripheral parenchymal atrophy, the size or weight of the liver, and obstructive portal vein thrombosis of the intrahepatic large portal veins and/or portal vein trunk. All of these factors are recognizable grossly as well as by imaging, radio-isotope scanning, peritoneoscopy, and/or portography. Evaluation of three parameters. Evaluation of the size or weight of liver: It is important to ascertain the weight of the IPH livers. Some livers were swollen, while others were markedly atrophic. The size of the liver is evaluable by imaging modalities, particularly computed tomography [12, 60]. Evaluation of subcapsular hepatic parenchymal atrophy: Subcapsular hepatic parenchymal atrophy is recognizable grossly on the cut surface of the autopsy liver. Magnetic resonance imaging (MRI) shows the proximity of medium-sized intrahepatic vessels to each other and to the liver surface [2, 3, 21]. Computed tomography (CT) during arterial portography shows abnormally short distances between some of the medium-sized portal branches and the liver surface [20]. Portography shows a lack of filling between the surface of the liver and peripheral vessels. Peripheral regional enhancement of the liver was seen in the arterial phase. On portograms taken via the superior mesenteric artery, markedly decreased portal venous perfusion was seen in the peripheral region of the liver. Evaluation of portal venous thrombosis: Assessment of portal vein patency and portal flow is most simply accomplished using Doppler ultrasonography, which also provides information about the hepatic veins and inferior vena cava. Portography of either kind is useful for the demonstration of occlusive portal veins. Proposal of a new staging system based on gross and imaging features. By a combination of these gross findings, IPH livers were categorized into four groups (Stage I, II, III, and IV) as shown below. Their schemes and likely progression are shown in Fig. 13. Stage I is early IPH, while stages II and III are advanced. To date, a staging system has not yet been described in the literature. This staging system can be applied to patients by combining imaging procedures with peritoneoscopy, and also to autopsy livers. In addition, the histological changes of IPH are known to be heterogeneous, and sampling errors are unavoidable, so the gross findings or imaging findings are considered more useful in staging. However, stage I cases are rare.
Proposal of a New Staging of IPH
It is generally thought that the IPH liver itself is already at the end-stage pathologically when diagnosed clinically. So, little is known about the pathological stages of IPH. However, as mentioned in the pathology of IPH livers, most pathological changes to IPH livers seem to be a consequence of a long-standing portal venous insufficiency in the liver. Therefore, these changes appear to progress and increase in their degree and extent during a long clinical course. Almost all chronic diseases of hepatocellular, biliary and hepatic venous obstructive diseases are known to be followed by the development of liver cirrhosis. Although the chronic effect of intrahepatic portal venous blocks on the liver may not lead to cirrhotic transformation, a pathological progression may evolve that may also be effective in IPH livers. By a combination of gross features, IPH livers could be grouped into several categories which may reflect the stage of the pathological progression of IPH or prognosis. The histological features of IPH should not be used as parameters for the pathological staging of IPH, because these are very heterogeneous, and sampling errors are likely to occur in needle or wedge biop-
74 Y. Nakanuma et al.
bolism of portal veins, but is more frequent in stage II and even more frequent at stage III. At autopsy, stage IV is relatively common. Superimposition of occlusive thrombosis of the intrahepatic large portal veins and/or portal vein trunk means a poor prognosis, and in fact, such patients frequently die of portal venous thrombosis. So, this factor was adopted as an independent staging factor in this system, for stage IV, when such a factor is found to be independent of other factors. In stage I, II and III livers, the intrahepatic large portal veins and extrahepatic portal veins show luminal thickening and even calcification, suggesting repeated thrombosis. As for stages I, II, and III, the histopathological changes may progress in this order. Portal venous occlusive thrombosis is likely to develop at advanced stages of IPH.
Conclusion
The recent progress in the study of IPH with respect to etiopathogenesis and pathology was reviewed. The proposal of a new staging system is based on the gross features of IPH, which reflect the secondary pathological features. The etiology could be multiple. It seems possible that IPH is heterogeneous in its initiation and/or progression depending on the etiology. The liver pathology characterized by occlusive changes of the intrahepatic portal radicles, portal and periportal fibrosis, and irregularly distributed parenchymal atrophies suggests some sort of portal venopathy that causes decreased portal perfusion of peripheral liver parenchyma. The most important issues of the pathobiology of IPH to be addressed and clarified in the near future are: 1) What are the etiologies? 2) How does portal hypertension develop and which pathological changes reflect the presinusoidal portal hypertension? and 3) How do secondary changes of the liver caused by intrahepatic hemodynamic alterations in IPH progress?
Acknowledgements. This study was supported by the Japanese Study Group of Intrahepatic Hemodynamic Alterations (Chairman: Prof. Keizo Sugimachi, Professor of Surgery, Kyushu University, Graduate School of Medicine, Fukuoka, Japan).
Fig. 13. Scheme of proposed staging of idiopathic portal hypertension. Stage I progresses to stage II, and stage II to stage III. In addition, each stage progresses to stage IV, characterized by occlusive portal venous thrombosis. Dotted areas beneath the hepatic capsule represent the subcapsular parenchymal atrophy and even collapse. The size of the liver is decreased at stage III. Phlebosclerosis of the hilar portal vein and of the intrahepatic large portal veins increases from stage I to stage II and from stage II to stage III both in degree and extent.
Stage I: Absence of peripheral parenchymal atrophy. The liver is usually not atrophic nor even swollen, and the surface is speculated to be smooth. IPH diagnosed at this stage is rare. Etiologic factor(s) remain to be identified at this stage. Stage II: Presence of peripheral parenchymal atrophy in non-atrophic liver. The surface is uneven or wavey and even vaguely nodular. The cut surface shows distinct subcapsular atrophy characterized by the close proximity of portal tracts or hepatic veins to the hepatic capsule. Stage III: Presence of peripheral parenchymal atrophy in atrophic liver. The surface is uneven or wavy, and even vaguely nodular. The cut surface shows distinct subcapsular atrophy. Occasionally, there is collapse involving areas or segments of the liver. Stage IV: Presence of obstructive thrombosis of intrahepatic large portal veins or portal vein trunk. This is regarded as being the superimposition of thromboem-
References
1. Aikat BK, Bhusnurmath SR, Chhuttani PN, Mitra SK, Dutta DV (1979) The pathology of noncirrhotic portal fibrosis: a review of 32 autopsy cases. Hum Pathol 10: 405418 2. Akaki S, Mitsumori A, Kanazawa S, Takeda Y, Joja I, Hiraki Y, Sakaguchi K (1997) Reduced radioactivity in the
Idiopathic Portal Hypertension 75 periphery of the liver in a patient with idiopathic portal hypertension. Clin Nucl Med 22: 369371 Arai K, Matsui O, Kadoya M, Yoshikawa J, Gabata T, Takashima T, Kobayashi K, Unoura M (1991) MR imaging in idiopathic portal hypertension. J Comput Assist Tomogr 15: 405408 Arakawa M, Kage M, Fukuta K (1988) Nodular lesion and portal hypertension. Report of intrahepatic hemodynamic alterations of Japanese Health and Welfare Minister, pp. 4447 Bernard PH, Le Bail B, Cransac M, Barcina MG, Carles J, Balabaud C, Bioulac-Sage P (1995) Progression from idiopathic portal hypertension to incomplete septal cirrhosis with liver failure requiring liver transplantation. J Hepatol 22: 495499 Bioulac-Sage P, Le Bail B, Bernard PH, Balabaud C. (1995) Hepatoportal sclerosis. Semin Liver Dis 15: 329339 Egesel T, Buyukasik Y, Dundar SV, Gurgey A, Kirazli S, Bayraktar Y (2000) The role of natural anticoagulant deficiencies and factor V Leiden in the development of idiopathic portal vein thrombosis. J Clin Gastroenterol 30: 6671 Fridman SL, Rockey DC, McGuire RF, Maher JJ, Boyles JK, Yamasaki G (1992) Isolated hepatic lipocytes and Kupffer cells from normal human liver: morphological and functional characteristics in primary culture. Hepatology 15: 234243 Futagawa S, Fukazawa M, Horisawa M, Musha H, Ito T, Sugiura M, Kameda H, Okuda K (1980) Portographic liver changes in idiopathic noncirrhotic portal hypertension. AJR Am J Roentgenol 134: 917923 Futagawa S, Fukazawa M, Musha H, Isomatsu T, Koyama K, Ito T, Horisawa M, Nakayama S, Sugiura M, Kameda H, Okuda K (1981) Hepatic venography in noncirrhotic idiopathic portal hypertension. Comparison with cirrhosis of the liver. Radiology 141: 303309 Greenwel P, Schwarz M, Peyrol S, Grimaud JA, Rojkind M (1991) Characterization of fat-storing cell lines derived from normal and Ccl4 cirrhosis livers: difference in the production of interleukin 6. Lab Invest 65: 644653 Heymsfield SB, Fulenwider T (1979) Accurate measurement of liver, kidney, and spleen volume and mass by computerized axial tomography. Ann Int Med 90: 185187 Huet PM, Guillaume E, Cote J, Legare A, Lavoie P, Viallet A (1975) Noncirrhotic presinusoidal portal hypertension associated with chronic arsenical intoxication. Gastroenterology 68: 12701277 Ichimura S, Sasaki R, Takemura Y, Iwata H, Obata H, Okuda H, Imai F (1993) The prognosis of idiopathic portal hypertension in Japan. Intern Med 32: 441444 Inagaki H, Nonami T, Kawagoe T, Miwa T, Hosono J, Kurokawa T, Harada A, Nakao A, Takagi H, Suzuki H, Sakamoto J (2000) Idiopathic portal hypertension associated with systemic lupus erythematosus. J Gastroenterol 35: 235239 Kerr JFR (1971) Shrinkage necrosis: a distinct mode of cellular death. J Pathol 105: 1320 Kobayashi S, Nakanuma Y, Matsui O (1994) Histopathology of portal tracts in livers after transcatheter arterial chemo-embolization therapy for hepatocellular carcinoma. J Gastroenterol Hepatol 9: 4554 Kono K, Ohnishi K, Omata M, Saito M, Nakayama T, Hatano H, Nakajima Y, Sugita S, Okuda K (1988) Experimental portal fibrosis produced by intraportal injection of killed nonpathogenic Escherichia coli in rabbits. Gastroenterology 94: 787796 Ludwig J, Hashimoto E, Obata H, Baldus WP (1993) Idiopathic portal hypertension; a histopathological study of 26 Japanese cases. Histopathology 22: 227234. Matsui O, Takashima T, Kadoya M, Kitagawa K (1984) Computed tomography during arterial portography in idiopathic portal hypertension. Radiat Med 2: 189193 Matsui O, Takashima T, Kadoya M, Kitagawa K (1984) Computed tomography during arterial portography in idiopathic portal hypertension. Radiat Med 2: 189193 Mikkelson WP, Edmondson HA, Peters RL, Redeker AG, Reynolds TB (1965) Extra- and intrahepatic portal hypertension without cirrhosis (hepatoportal sclerosis). Ann Surg 162: 602620 Mori K, Ishimura E, Goto H, Shoji S, Seki S, Yamashita T, Wakasa K, Nishizawa Y, Morii H (1996) Idiopathic portal hypertension associated with high serum titer of autoantibodies and liver dysfunction. J Gastroenterol 31: 123128 Nakak NC, Ramalingaswami V (1969) Obliterative portal venopathy of the liver associated with so-called idiopathic portal hypertension or tropical splenomegaly. Arch Pathol 87: 359369 Nakanuma Y, Hoso M, Sasaki M, Terada T, Katayanagi K, Nonomura A, Kurumaya H, Harada A, Obata H (1996) Histopathology of the liver in non-cirrhotic portal hypertension of unknown aetiology. Histopathology 28: 195204 Nakanuma Y, Nonomura A, Hayashi M, Doishita K, Takayanagi N, Uchida T, Obata Y, Noma K, Ikoma J, Yoshikawa K, et al. (1989) Pathology of the liver in "idiopathic portal hypertension" associated with autoimmune disease. The Ministry of Health and Welfare Disorders of Portal Circulation Research Committee. Acta Pathol Jpn 39: 586592 Nakanuma Y, Ohta G, Takeshita H, Yamazaki Y, Doishita K, Shimizu M (1983) Florid duct lesions and extensive bile duct loss of the intrahepatic biliary tree in chronic liver diseases other than primary biliary cirrhosis. Acta Pathol Jpn 33: 10951104 Nayyar AK, Sharma BK, Sarin SK, Malhotra P, Broor SL, Sachdev G (1990) Characterization of peripheral blood lymphocytes in patients with non-cirrhotic portal fibrosis: a comparison with cirrhotics and healthy controls. J Gastroenterol Hepatol 5: 554559. Ohbu M, Okudaira M (1996) Subintimal edematous and cellular lesions of intrahpeatic portal veins in portal hypertension. Report of intrahepatic hemodynamic alterations of Japanese Health and Welfare Minister, pp. 3237 Ohbu M, Okudaira M, Watanabe K, Kaneko S, Takai T (1994) Histopathological study of intrahepatic aberrant vessels in cases of noncirrhotic portal hypertension. Hepatology 20: 302308 Ohnishi K, Chin N, Tanaka H, Iida S, Sato S, Terabayashi H, Nomura F (1989) Differences in portal hemodynamics in cirrhosis and idiopathic portal hypertension. Am J Gastroenterol 84: 409412 Ohnishi K, Saito M, Sato S, Terabayashi H, Iida S, Nomura F, Nakano M, Okuda K (1987) Portal hemodynamics in idiopathic portal hypertension (Bantis syndrome). Comparison with chronic persistent hepatitis and normal subjects. Gastroenterology 92: 751758
3.
19. 20. 21. 22.
4.
5.
6. 7.
23.
24.
8.
25.
9.
26.
10.
11.
27.
12. 13.
28.
29.
14. 15.
30.
31.
16. 17.
32.
18.
76 Y. Nakanuma et al. 33. Oikawa H, Masuda T, Sato S, Yashima A, Suzuki K, Sato S, Satodate R (1998) Changes in lymph vessels and portal veins in the portal tract of patients with idiopathic portal hypertension: a morphometric study. Hepatology 27: 16071610 34. Okuda K (1989) Idiopathic portal hypertension and protein C deficiency. Hepatology 10: 903 35. Okuda K, Kono K, Ohnishi K, Kimura K, Omata M, Koen H, Nakajima Y, Musha H, Hirashima T, Takashi M, et al. (1984) Clinical study of eighty-six cases of idiopathic portal hypertension and comparison with cirrhosis with splenomegaly.Gastroenterology 86: 600610 36. Okuda K, Nakashima T, Okudaira M, Kage M, Aida Y, Omata M, Sugiura M, Kameda H, Inokuchi K, Bhusnurmath SR, Aikat BA (1982) Liver pathology of idiopathic portal hypertension. Comparison with non-cirrhotic portal fibrosis of India. The Japan idiopathic portal hypertension study. Liver 2: 176192 37. Okuda K, Ohnishi K, Kimura K, Matsutani S, Sumida M, Goto N, Musha H, Takashi M, Suzuki N, Shinagawa T, et al. (1985) Incidence of portal vein thrombosis in liver cirrhosis. An angiographic study in 708 patients. Gastroenterology 89: 279286 38. Onitsuka A, Yano Y, Yamada N, Tanabe H, Hino A, Ozeki Y, Hayashi M, Horiya Y, Goto A (1984) Case report of idiopathic portal hypertension with Cruveilhier-Baumgarten syndrome and portal thrombosis. Nippon Shokakibyo Gakkai Zasshi 81: 14851488 39. Orozco H, Guraieb E, Takahashi T, Garcia-Tsao G, Hurtado R, Anaya R, Ruiz-Arguelles G, Hernandez-Ortiz J, Casillas MA, Guevara L (1988) Deficiency of protein C in patients with portal vein thrombosis. Hepatology 8: 11101111 40. Paradis V, Dargere D, Vidaud M, De Gouville AC, Huet S, Martinez V, Gauthier JM, Ba N, Sobesky R, Ratziu V, Bedossa P (1999) Expression of connective tissue growth factor in experimental rat and human liver fibrosis. Hepatology 30: 968976 41. Ramadori G (1991) The stellate cell (Ito cell, fat-storing cell, lipocyte, perisinusoid cell) of the liver: new insight into pathophysiology of an intriguing cell. Virchow Archiv [B] 61: 147151 42. Saito K, Nakanuma Y, Takegoshi K, Ohta G, Obata Y, Okuda K, Kameda H (1993) Non-specific immunological abnormalities and association of autoimmune diseases in idiopathic portal hypertension. A study by questionnaire. Hepatogastroenterology 40: 163166 43. Sato S, Nagaoka T, Hasegawa M, Tamatani T, Nakanishi T, Takigawa M, Takehara K (2000) Serum levels of connective tissue growth factor are elevated in patients with systemic sclerosis: association with extent of skin sclerosis and severity of pulmonary fibrosis. J Rheumatol 27: 149154 44. Schmitt-Graff A, Kruger S, Bochard F, Gabbiani G, Denk H (1991) Modulation of alpha smooth muscle actin and desmin expression in perisinusoidal cells of normal and diseased human livers. Am J Pathol 138: 12331242 45. Sekiguchi Y, Amano K, Takano Y, Saito K, Itoh I, Tsuzaka K, Abe T, Takeuchi T (1999) Portal and pulmonary hypertension in a patient with MCTD. Ryumachi 39: 657663 (in Japanese) 46. Shepherd P, Harrison DJ (1990) Idiopathic portal hypertension associated with cytotoxic drugs. J Clin Pathol 43: 206210 47. Shimamatsu K, Wanless IR (1997) Role of ischemia in causing apoptosis, atrophy, and nodular hyperplasia in human liver. Hepatology 26: 343350 48. Sugimura T, Tsuji Y, Ibayashi H, Sakai H, Nawata H (1996) Portal-systemic shunting in a patient with normal portal vain pressures and histological evidence of idiopathic portal hypertension. J Gastroenterol Hepatol 11: 301304 49. Sugita S, Ohnishi K, Iida S, Nomura F, Okuda K (1989) Histological changes in the liver and portal hypertension subsequent to repeated intraportal injections of killed E. coli in the dog. Liver 8: 19 50. Terada T, Nakanuma Y, Hoso M, Obata H (1991) Expression of HLA-DR antigen on hepatic vascular endothelial cells in idiopathic portal hypertension. Clin Exp Immunol 84: 303307 51. Terada T, Nakanuma Y, Obata H (1991) HLA-DR expression on the microvasculature of portal tracts in idiopathic portal hypertension. Immunohistochemical characteristics and relation to portal phlebosclerosis. Arch Pathol Lab Med 115: 993997 52. Thomas LB, Popper H, Berk PD, Selikoff I, Falk H (1975) Vinyl-chloride-induced liver disease. From idiopathic portal hypertension (Bantis syndrome) to Angiosarcomas. N Engl J Med 292: 1722 53. Vakili C, Farahvash MJ, Bynum TE (1992) Endemic idiopathic portal hypertension: report on 32 patients with non-cirrhotic portal fibrosis. World J Surg 16: 118124 54. Wanless IR (1994) Vascular disorders. In: MacSween RNM, Anthony PP, Scheuer PJ, Burt AD, Portmann BC (Eds) Pathology of the liver, 3rd ed., pp. 535562. Churchill Livingstone Inc, Edinburgh 55. Wanless IR, Godwin TA, Allen F, Feder A (1980) Nodular regenerative hyperplasia of the liver in hematologic disorders: a possible response to obliterative portal venopathy. A morphometric study of nine cases with an hypothesis on the pathogenesis. Medicine (Baltimore) 59: 367379 56. Wanless IR, Peterson P, Das A, Boitnott JK, Moore GW, Bernier V (1990) Hepatic vascular disease and portal hypertension in polycythemia vera and agnogenic myeloid metaplasia: a clinicopathological study of 145 patients examined at autopsy. Hepatology 12: 11661174 57. Wyllie AH (1987) Apoptosis: cell death in tissue regulation. J Pathol 153: 313316 58. Yamaguchi N, Tokushige K, Haruta I, Yamauchi K, Hayashi N (1999) Analysis of adhesion molecules in patients with idiopathic portal hypertension. J Gastroenterol Hepatol 14: 364369 59. Yamaoka K, Nouchi T, Marumo F, Sato C (1993) Alphasmooth-muscle actin expression in normal and fibrotic human livers. Digestive Diseases and Science 38: 14731479 60. Yoshida K, Kato H, Tsukada K, Muto K (1990) Liver volume after different therapies. Report of intrahepatic hemodynamic alterations of Japanese Health and Welfare Minister, pp. 175177 61. Yoshimura N, Oka T, Ohmori Y, Yasumura T, Kohnosu H, Kobashi T (1994) Idiopathic portal hypertension in renal transplant recipients: report of two cases. Surg Today 24: 11111114 Received: October 10, 2000 Accepted: October 16, 2000
You might also like
- Portal HypertensionDocument65 pagesPortal HypertensionVenu MadhavNo ratings yet
- Oxford Textbook of Medicine, 5th EdDocument101 pagesOxford Textbook of Medicine, 5th EdIzni Zinin75% (4)
- Altered Pharmacokinetics in Liver DiseasesDocument30 pagesAltered Pharmacokinetics in Liver DiseasesNailaAns100% (1)
- Hepatobiliary DiseaseDocument60 pagesHepatobiliary DiseaseFirdaus AslamNo ratings yet
- Seminar On Cirrhosis of LiverDocument9 pagesSeminar On Cirrhosis of Liverrufusprasanth_rachaproluNo ratings yet
- Single Best Answer (SBA) QuestionsDocument16 pagesSingle Best Answer (SBA) QuestionsKrairat Komdee50% (2)
- Portal HypertensionDocument41 pagesPortal Hypertensionams_1234100% (2)
- Hepatic EncephalopathyDocument2 pagesHepatic Encephalopathyjustifieda13No ratings yet
- Medical Surgical NursingDocument31 pagesMedical Surgical Nursingsingireddi1revathi100% (3)
- Diagnosis of Portal Hypertension 1Document20 pagesDiagnosis of Portal Hypertension 1sussie jeffersonNo ratings yet
- Liver Transplant - PedsDocument15 pagesLiver Transplant - Pedsbencleese100% (2)
- Fitz Abdominal Paces NotesDocument19 pagesFitz Abdominal Paces NotesDrShamshad Khan100% (1)
- Portalhypertensivegastropathy Andcolopathy: Nathalie H. Urrunaga,, Don C. RockeyDocument18 pagesPortalhypertensivegastropathy Andcolopathy: Nathalie H. Urrunaga,, Don C. RockeyshahirahrasidNo ratings yet
- 09 Portal HypertensionDocument51 pages09 Portal HypertensionAna TudosieNo ratings yet
- Non Invasive Evaluation Using Ultrasound Elastography For Portal HypertensionDocument13 pagesNon Invasive Evaluation Using Ultrasound Elastography For Portal HypertensionRendyNo ratings yet
- Hipertension Portal.Document15 pagesHipertension Portal.Andres BernalNo ratings yet
- HP - Patogenesis y DiagnosticoDocument15 pagesHP - Patogenesis y DiagnosticoJEAN QUISPENo ratings yet
- Portal Hypertension: Pathophysiology, Diagnosis and ManagementDocument11 pagesPortal Hypertension: Pathophysiology, Diagnosis and ManagementXol-TikaNo ratings yet
- Anaesth For TipsDocument8 pagesAnaesth For TipsNitasha RoyNo ratings yet
- B9781416049197X50014 B978141604919750178X MainDocument4 pagesB9781416049197X50014 B978141604919750178X MainMANGNo ratings yet
- Ascitesandhepatorenal Syndrome: Danielle Adebayo,, Shuet Fong Neong,, Florence WongDocument24 pagesAscitesandhepatorenal Syndrome: Danielle Adebayo,, Shuet Fong Neong,, Florence WongHernan GonzalezNo ratings yet
- Portal Hypertension Pathogenesis and Diagnosis PDFDocument15 pagesPortal Hypertension Pathogenesis and Diagnosis PDFLizeth GirónNo ratings yet
- EN Esophageal Varices Bleeding in Portal Hy PDFDocument4 pagesEN Esophageal Varices Bleeding in Portal Hy PDFYuko Ade WahyuniNo ratings yet
- Left-Sided Portal Hypertension: A Clinical Challenge: Hipertensão Portal Esquerda: Um Desafio ClínicoDocument3 pagesLeft-Sided Portal Hypertension: A Clinical Challenge: Hipertensão Portal Esquerda: Um Desafio ClínicomichaelqurtisNo ratings yet
- 1481 5201 1 PBDocument9 pages1481 5201 1 PBChiperi CristinaNo ratings yet
- OLGU HHT 13 ŞubatDocument9 pagesOLGU HHT 13 ŞubatAli Uğur SoysalNo ratings yet
- Imaging in Cirrhosis PDFDocument51 pagesImaging in Cirrhosis PDFranjithajayNo ratings yet
- Pediatric Living Donor Liver Transplantation WithDocument21 pagesPediatric Living Donor Liver Transplantation WithAngger SatriaNo ratings yet
- Jurnal Non Sirotic VEDocument21 pagesJurnal Non Sirotic VEDheajeng Intan MutiarasariNo ratings yet
- № 15. PM with hepatolienal syndrome, portal hypertension, jaund - Яссін КумаілDocument8 pages№ 15. PM with hepatolienal syndrome, portal hypertension, jaund - Яссін Кумаілabo salehNo ratings yet
- Khanna 2018Document20 pagesKhanna 2018wahid akbarNo ratings yet
- 2015 Article 79Document6 pages2015 Article 79Alfeus GradyNo ratings yet
- Uworld GI NotesDocument17 pagesUworld GI NotesAyodeji SotimehinNo ratings yet
- Portal Hypertension: IntroductionDocument13 pagesPortal Hypertension: IntroductionGaoudam NatarajanNo ratings yet
- Jo Boy With SOB 04202016Document25 pagesJo Boy With SOB 04202016sirrfsNo ratings yet
- Portopulmonary Hypertension and Hepatopulmonary Syndrome: ReviewDocument8 pagesPortopulmonary Hypertension and Hepatopulmonary Syndrome: ReviewAdel HamadaNo ratings yet
- Portal HypertensionDocument11 pagesPortal Hypertensionsaeid seyedraoufiNo ratings yet
- Esophageal Varices: Pathophysiology, Approach, and Clinical DilemmasDocument2 pagesEsophageal Varices: Pathophysiology, Approach, and Clinical Dilemmaskaychi zNo ratings yet
- An Unusual Case of Polycythemia Vera With A Complication of Pancreatic PseudocystDocument3 pagesAn Unusual Case of Polycythemia Vera With A Complication of Pancreatic PseudocystAgus PrimaNo ratings yet
- A 2 Year Old Boy With Hypoxemia, Pulmonary HyperteDocument4 pagesA 2 Year Old Boy With Hypoxemia, Pulmonary HyperteagamerocallejasNo ratings yet
- Causes and Pathomechanisms of Oesophageal Varices DevelopmentDocument15 pagesCauses and Pathomechanisms of Oesophageal Varices DevelopmentYeffersonMelendezNo ratings yet
- B.SC Nursing Medical Surgical Nursing - I Unit: Iv - Nursing Management of Patients With Disorders of Digestive System Portal HypertensionDocument32 pagesB.SC Nursing Medical Surgical Nursing - I Unit: Iv - Nursing Management of Patients With Disorders of Digestive System Portal HypertensionPoova RagavanNo ratings yet
- Cirrosis LANCET 2008Document14 pagesCirrosis LANCET 2008Natalia ElizabethNo ratings yet
- Imagen Hepática Vascular Hígado 2022Document15 pagesImagen Hepática Vascular Hígado 2022Oscar BushNo ratings yet
- 8156 29309 1 PBDocument5 pages8156 29309 1 PBEden LacsonNo ratings yet
- Liver Transplant Workup: IntroductionDocument9 pagesLiver Transplant Workup: IntroductionKay BristolNo ratings yet
- Portal HypertensionDocument13 pagesPortal HypertensionCiprian BoesanNo ratings yet
- Liver CirrhosisDocument9 pagesLiver CirrhosismedsmracelisNo ratings yet
- Hipertencion PortalDocument4 pagesHipertencion PortalLina Isabella MurgasNo ratings yet
- (12205818 - Internal Medicine) Gave Syndrome - A Rare and Mysterious Cause of Gastrointestinal Hemorrhage in The ElderlyDocument10 pages(12205818 - Internal Medicine) Gave Syndrome - A Rare and Mysterious Cause of Gastrointestinal Hemorrhage in The ElderlysimonaNo ratings yet
- Left SidedDocument3 pagesLeft SidedElisabeth ZzMick GtNo ratings yet
- Imaging of The Porta HepatisDocument71 pagesImaging of The Porta Hepatisjskmkabongo100% (1)
- Management of Hyponatremia in Clinical Hepatology Practice: Liver (B Bacon, Section Editor)Document5 pagesManagement of Hyponatremia in Clinical Hepatology Practice: Liver (B Bacon, Section Editor)deltanueveNo ratings yet
- Application of Ultrasound Elastography in Assesing Portal HypertensionDocument16 pagesApplication of Ultrasound Elastography in Assesing Portal HypertensionValentina IorgaNo ratings yet
- Chronic Liver DiseasesDocument43 pagesChronic Liver DiseasesDickson Luvi100% (1)
- Portal Hypertension: SymposiumDocument4 pagesPortal Hypertension: SymposiumIsrael BlancoNo ratings yet
- Acute Liver Failure-1Document40 pagesAcute Liver Failure-1elizabethNo ratings yet
- W J C M P R: Orld Ournal of Urrent Edical and Harmaceutical EsearchDocument3 pagesW J C M P R: Orld Ournal of Urrent Edical and Harmaceutical EsearchVivi DeviyanaNo ratings yet
- HcirhosisDocument11 pagesHcirhosisChetendra IndoliaNo ratings yet
- Amjcaserep 16 751Document5 pagesAmjcaserep 16 751Chetan DhobleNo ratings yet
- Portal Hypertension SPDocument19 pagesPortal Hypertension SPAshish SatyalNo ratings yet
- Pathohysiology of Ascites: Waleed Al-HamoudiDocument36 pagesPathohysiology of Ascites: Waleed Al-Hamoudiarina31No ratings yet
- Ischemic Hepatitis, Hepatic Infarction, and Ischemic Cholangiopathy - UpToDateDocument9 pagesIschemic Hepatitis, Hepatic Infarction, and Ischemic Cholangiopathy - UpToDateAmirhossein MemariNo ratings yet
- Cirro SisDocument13 pagesCirro Sisbruno baileyNo ratings yet
- Abnormal Hematological Indices in CirrhosisDocument6 pagesAbnormal Hematological Indices in Cirrhosismy accountNo ratings yet
- Pulmonary Interstitial/Vascular Involvement: In-Depth Discussion IiDocument12 pagesPulmonary Interstitial/Vascular Involvement: In-Depth Discussion IiAna-Maria IoniţăNo ratings yet
- Gi 4Document3 pagesGi 4Syifa' FauziyahNo ratings yet
- The Evolving Landscape of Liver Cirrhosis ManagementFrom EverandThe Evolving Landscape of Liver Cirrhosis ManagementHitoshi YoshijiNo ratings yet
- 1 An Evolutionary Perspective On The Origin REVIEWDocument9 pages1 An Evolutionary Perspective On The Origin REVIEWJuan Camilo MoralesNo ratings yet
- J10 - T3 Criterios de Cotton ColedocolitiasisDocument7 pagesJ10 - T3 Criterios de Cotton ColedocolitiasisJuan Camilo MoralesNo ratings yet
- Radiological Investigation in Acute Diverticulitis: ReviewDocument6 pagesRadiological Investigation in Acute Diverticulitis: ReviewJuan Camilo MoralesNo ratings yet
- Book Reviews: R e N A L Disease in Children - Clinical Evaluation and DiagnosisDocument1 pageBook Reviews: R e N A L Disease in Children - Clinical Evaluation and DiagnosisJuan Camilo MoralesNo ratings yet
- What Is Alcohol2012AAlisherMDPhDDocument58 pagesWhat Is Alcohol2012AAlisherMDPhDAlisher AgzamovNo ratings yet
- Preparing For The NASH Epidemic A Call To ActioDocument11 pagesPreparing For The NASH Epidemic A Call To ActioDaniNo ratings yet
- Digestive Disorders in Tortoises From Vetlexicon ReptileDocument4 pagesDigestive Disorders in Tortoises From Vetlexicon Reptilemomo689No ratings yet
- Management of Chronic Hepatitis B in AdultsDocument76 pagesManagement of Chronic Hepatitis B in AdultsLan AllemNo ratings yet
- Management of Splenic Injury in The Adult Trauma PatientDocument17 pagesManagement of Splenic Injury in The Adult Trauma PatientArnaldo Santizo SáenzNo ratings yet
- MCLD 2020 - Poster Board NumberDocument3 pagesMCLD 2020 - Poster Board NumberArdianto SucintaNo ratings yet
- ACLF in ICUDocument42 pagesACLF in ICUEric CharpentierNo ratings yet
- Hepb Evidence ReportDocument671 pagesHepb Evidence ReportHerly Maulida SurdhawatiNo ratings yet
- Hepatitis C in PregnancyDocument9 pagesHepatitis C in PregnancychanyundaNo ratings yet
- J Cellular Molecular Medi - 2014 - Liu - The Multiple Functional Roles of Mesenchymal Stem Cells in Participating inDocument10 pagesJ Cellular Molecular Medi - 2014 - Liu - The Multiple Functional Roles of Mesenchymal Stem Cells in Participating indr. RiyanNo ratings yet
- Hepatitis GeneralDocument14 pagesHepatitis GeneralMarysol UlloaNo ratings yet
- Baveno VI Consensus Workshop 2015 PDFDocument10 pagesBaveno VI Consensus Workshop 2015 PDFMadalina StoicescuNo ratings yet
- Different Energy Sources of Liver ResectionDocument13 pagesDifferent Energy Sources of Liver ResectionAnirban Ghosh100% (1)
- Hepatic Disorders: Michael D. Manglapus, RN, RM, MANDocument108 pagesHepatic Disorders: Michael D. Manglapus, RN, RM, MANMichael Baylon DueñasNo ratings yet
- Short Cases: Gastroenterology Part 2 Exam PrepDocument48 pagesShort Cases: Gastroenterology Part 2 Exam PrepMobeen RazaNo ratings yet
- 述评 Human Albumin Infusion Strategy in Liver Cirrhosis Liberal or RestrictiveDocument3 pages述评 Human Albumin Infusion Strategy in Liver Cirrhosis Liberal or Restrictive倪沁赟No ratings yet
- Alcoholic Liver DiseaseDocument22 pagesAlcoholic Liver DiseaseRaju NiraulaNo ratings yet
- UGIB Prob Sec To BPUD Vs Gastric Mass Alzheimer's DiseaseDocument7 pagesUGIB Prob Sec To BPUD Vs Gastric Mass Alzheimer's DiseaseMonique Angela Turingan GanganNo ratings yet
- Fibroscan 01Document8 pagesFibroscan 01AmiablePCNo ratings yet
- Developmental Endocrine Reproductive Toxicologist in NJ NY Resume Edward FrizellDocument7 pagesDevelopmental Endocrine Reproductive Toxicologist in NJ NY Resume Edward FrizellEdwardFrizellNo ratings yet
- Chapter 25Document27 pagesChapter 25Kriana RosalesNo ratings yet