Professional Documents
Culture Documents
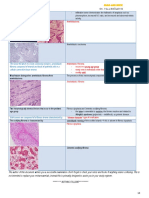
Choroidal Melanoma
Choroidal Melanoma
Uploaded by
Peter AbikoyeCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Choroidal Melanoma
Choroidal Melanoma
Uploaded by
Peter AbikoyeCopyright:
Available Formats
Pathophysiology Primary choroidal melanoma arises from melanocytes within the choroid.
Most choroidal melanomas are believed to develop from preexisting melanocytic nevi, though de novo growth of choroidal melanomas also occurs. Three distinct cell types are recognized in choroidal and other uveal melanomas: (1) spindle A, (2) spindle B, and (3) epithelioid. The last cell type usually has the most aggressive behavior and carries a poorer prognosis for the patients long-term survival. Choroidal melanomas may have variable coloration, ranging from darkly pigmented to purely amelanotic. They typically are dome-shaped. As they enlarge, if they break through the Bruch membrane, they can assume a mushroom configuration. Other shapes found for these tumors are bilobular, multilobular, and diffuse. The last of these is characterized by lateral growth throughout the choroid with minimal elevation; it occurs in about 5% of cases. Rarely, choroidal melanomas may arise in a multicentric distribution in 1 or both eyes. Choroidal melanomas affect the retinal pigment epithelium as they push against it and deprive it of normal choroidal circulation. Overlying retinal pigment epithelium usually develops areas of atrophy, drusen, and localized pigment epithelial detachments. Areas of phagocytic activity, where cellular debris from melanocytes is digested, give the pigment epithelium patches of coloration change. Macrophages within these typically orange areas contain melanin and lipofuscin. These changes can lead to choroidal neovascularization over the tumor, with consequent subretinal exudation, hemorrhage, and fibrous plaque formation. Growth of choroidal melanomas can occur silently until it produces enough visual loss through various mechanisms.[3] The tumors disruption of choroidal circulation and consequent ischemia typically cause degeneration of retinal photoreceptors and other retinal neurons. The retina overlying the tumor can separate into cystoid spaces and larger schisis cavities. There may be associated cystoid macular edema. In general, the farther the tumors origin is from the optic nerve and fovea, the larger the tumor can become before the patient notices a visual field defect. Exudation of fluid into the subretinal space with consequent retinal detachment may enlarge the field loss. This exudation can lead to total retinal detachment. Rarely, choroidal melanomas can impinge into underlying posterior ciliary nerves, causing severe ocular pain. Other signs and symptoms can result if the tumor grows anteriorly, pathologically involving the ciliary body, trabecular meshwork, and lens, with consequent ocular hypotension or hypertension and cataract. Large choroidal melanomas can induce iris rubeosis. Erosion of the melanoma into blood vessels in adjacent tissues, or areas of necrosis within the tumor, can lead to vitreous hemorrhage or hyphema. Choroidal melanoma ultimately causes death, practically always secondary to distant metastases rather than local spread. Its metastatic potential depends on the histopathologic aggressiveness of the tumor cells. Unfortunately, it not infrequently metastasizes before diagnosis. If the melanoma does not show extraocular extension, it can only spread hematogenously, because there are no lymphatic vessels in the eye. It most often metastasizes to the liver; other organs of dissemination include the lung, bone, skin, and central nervous system (CNS). Less frequently, choroidal melanoma can grow transsclerally, through emissary channels, and metastasize locally into the orbit or rarely the conjunctiva. Choroidal melanoma almost never extends through the optic nerve; when it does, it is usually in juxtapapillary tumors or in diffuse choroidal melanomas. References
You might also like
- HEAD AND NECK 1.robbins & Cotran Pathologic Basis of Disease ReviewerDocument14 pagesHEAD AND NECK 1.robbins & Cotran Pathologic Basis of Disease ReviewerSeff Causapin100% (1)
- Squamous Cell Carcinoma-Well DifferentiatedDocument4 pagesSquamous Cell Carcinoma-Well DifferentiatedYukankolmi OyoNo ratings yet
- MeningiomaDocument7 pagesMeningiomaLili HapverNo ratings yet
- CVS Essay QuestionsDocument3 pagesCVS Essay QuestionsPeter AbikoyeNo ratings yet
- Systemic and Localized Scleroderm11!05!08Document99 pagesSystemic and Localized Scleroderm11!05!08Linux LinuxNo ratings yet
- Dermatology Notes for Medical StudentsFrom EverandDermatology Notes for Medical StudentsRating: 4 out of 5 stars4/5 (5)
- Masquerade SyndromeDocument69 pagesMasquerade Syndromerohitaswa100% (1)
- MnemonicS in OphthaDocument22 pagesMnemonicS in Ophthaabuahmed&janaNo ratings yet
- Dermatology Essay QuestionsDocument2 pagesDermatology Essay QuestionsPeter Abikoye100% (1)
- MCQS CNS PathologyDocument14 pagesMCQS CNS PathologyFourth YearNo ratings yet
- Skin Cancer ShowDocument57 pagesSkin Cancer ShowNice YouNo ratings yet
- Urology Essay QuestionsDocument2 pagesUrology Essay QuestionsPeter AbikoyeNo ratings yet
- Management Vascular MalformationDocument18 pagesManagement Vascular MalformationRini RahmawulandariNo ratings yet
- Basal Cell Carcinoma - Pathophysiology and ManagementDocument6 pagesBasal Cell Carcinoma - Pathophysiology and ManagementReylan Garcia0% (1)
- 07 BMDocument9 pages07 BMMajid KhanNo ratings yet
- Airway Assessment MeDocument46 pagesAirway Assessment MePeter AbikoyeNo ratings yet
- Forensics Essay QuestionsDocument2 pagesForensics Essay QuestionsPeter Abikoye100% (2)
- Neurology Essay QuestionsDocument3 pagesNeurology Essay QuestionsPeter Abikoye100% (3)
- Dr. C.T. Karthikeyan, Associate Professor, Dept. of General SurgeryDocument40 pagesDr. C.T. Karthikeyan, Associate Professor, Dept. of General SurgeryNaveen RajeshwarNo ratings yet
- 6 - Disorders of MelanocytesDocument5 pages6 - Disorders of MelanocytesAbdul FatahNo ratings yet
- Eye Pathology: Dr. Jusuf FantoniDocument8 pagesEye Pathology: Dr. Jusuf Fantonitutor tujuhNo ratings yet
- LEUKOKORIADocument3 pagesLEUKOKORIAFahlevie EpinNo ratings yet
- Skin Cancers: Assistant Larisa PoroshinaDocument42 pagesSkin Cancers: Assistant Larisa PoroshinaMed PoxNo ratings yet
- Oculars TumorDocument109 pagesOculars TumorNovita EmyNo ratings yet
- Peripheral Central Giant Cell Granuloma NXPowerLiteDocument18 pagesPeripheral Central Giant Cell Granuloma NXPowerLiteAFREEN SADAF100% (1)
- TumourDocument7 pagesTumourعلي احمد جواد حسينNo ratings yet
- Skin TumDocument9 pagesSkin TumElsa OctaviaNo ratings yet
- TUMOR MATA FX TranslateDocument34 pagesTUMOR MATA FX TranslateAisyahNo ratings yet
- Limbal DermoidDocument4 pagesLimbal DermoidPranjali ChhayaNo ratings yet
- Tumor of The EyeDocument40 pagesTumor of The EyeGustiAngriAngalan100% (1)
- Premalignant and Malignant Skin DiseasesDocument25 pagesPremalignant and Malignant Skin Diseasesmedical1acc2No ratings yet
- Fibrosarcoma Differential DiagnosisDocument10 pagesFibrosarcoma Differential DiagnosisdmdsahNo ratings yet
- Pediatric Tumors of The Eye and OrbitDocument60 pagesPediatric Tumors of The Eye and OrbitDMdewiNo ratings yet
- Conway Lien, MD Mahesh R Patel, MDDocument8 pagesConway Lien, MD Mahesh R Patel, MDBoby SuryawanNo ratings yet
- Skin CancerDocument7 pagesSkin Cancerعبدالعزيز احمد علي عتشNo ratings yet
- Retinal Hemangiomas - American Academy of OphthalmologyDocument10 pagesRetinal Hemangiomas - American Academy of OphthalmologyLydia Angelia YanitaNo ratings yet
- History: Imaging StudiesDocument5 pagesHistory: Imaging StudiesHerdyastuti NurwestriNo ratings yet
- Skin CancerDocument5 pagesSkin CancerEl FaroukNo ratings yet
- 21 Benign Skin TumorsDocument4 pages21 Benign Skin TumorsAbdul Ghaffar AbdullahNo ratings yet
- PATHOLOGYDocument188 pagesPATHOLOGYMaisha Maliha ShamsNo ratings yet
- Tumor Suzne Zlijezde AAODocument4 pagesTumor Suzne Zlijezde AAOJovan PopovićNo ratings yet
- 2.11.1 Benign and Malignant Skin LesionsDocument14 pages2.11.1 Benign and Malignant Skin LesionsZayan SyedNo ratings yet
- Pulmonary Metastasis 2Document7 pagesPulmonary Metastasis 2raisamentariNo ratings yet
- CNS Flash PointsDocument3 pagesCNS Flash PointsAbdul MannanNo ratings yet
- Seminar Presentation On Common Skin Tumors: - Mekonnen (RII) - Moderator Dr. Teka Consultant Surgeon)Document61 pagesSeminar Presentation On Common Skin Tumors: - Mekonnen (RII) - Moderator Dr. Teka Consultant Surgeon)moges beletachawNo ratings yet
- Malignant Epithelial Non-Odontogenic Tumors 2Document11 pagesMalignant Epithelial Non-Odontogenic Tumors 2samamustafa.2003No ratings yet
- Conjunctival Pigmented LesionsDocument0 pagesConjunctival Pigmented LesionsBhartendu Agarwal0% (1)
- Patologi MataDocument30 pagesPatologi Matasiti agusriantinaNo ratings yet
- Eye TermsDocument16 pagesEye TermsTa Thuy LinhNo ratings yet
- DBST Dlja INO Studentov (PDF - Io)Document6 pagesDBST Dlja INO Studentov (PDF - Io)eidNo ratings yet
- Tumors of The Eye: Antony Halim I4061162030Document64 pagesTumors of The Eye: Antony Halim I4061162030Bella Faradiska YuandaNo ratings yet
- Tumors of The CNSDocument26 pagesTumors of The CNSShailendra Pratap SinghNo ratings yet
- Soft Tissue TumorDocument67 pagesSoft Tissue TumorVincentius Michael WilliantoNo ratings yet
- Orbital ExenterationDocument9 pagesOrbital ExenterationJoji Dela pPeñaNo ratings yet
- RomJOphthalmol 59 74Document4 pagesRomJOphthalmol 59 74Dilshan DissanayakaNo ratings yet
- Chapter 455 Retinoblastoma Retinoblastoma Charles B. Pratt: PathologyDocument4 pagesChapter 455 Retinoblastoma Retinoblastoma Charles B. Pratt: PathologyEbook Kedokteran Bahan KuliahNo ratings yet
- CASE Report Basal Cell Carsinoma of NoseDocument22 pagesCASE Report Basal Cell Carsinoma of NoseDestar Aditya SadegaNo ratings yet
- Lec. 8 Malignant Bone TumorsDocument17 pagesLec. 8 Malignant Bone Tumorsنور كاضمNo ratings yet
- Lab 4Document10 pagesLab 4medical.student.messiNo ratings yet
- Skin Pigmentation + Hari DisorderDocument113 pagesSkin Pigmentation + Hari DisorderAfiqah So JasmiNo ratings yet
- Glomerular Diseases: Ass - Prof. Rihab Al-Mudhaffer Kufa University Department of Pathology and Forensic MedicineDocument37 pagesGlomerular Diseases: Ass - Prof. Rihab Al-Mudhaffer Kufa University Department of Pathology and Forensic MedicineAli HusseinNo ratings yet
- AMC Handbook NotesDocument4 pagesAMC Handbook Notesdr_navsterNo ratings yet
- Feline Diffuse Iridal Melanoma: PresentationDocument23 pagesFeline Diffuse Iridal Melanoma: PresentationJosh LittleNo ratings yet
- Presentation 1Document31 pagesPresentation 1Nice YouNo ratings yet
- Fast Facts: Advanced Cutaneous Squamous Cell Carcinoma for Patients and their Supporters: Information + Taking Control = Best OutcomeFrom EverandFast Facts: Advanced Cutaneous Squamous Cell Carcinoma for Patients and their Supporters: Information + Taking Control = Best OutcomeNo ratings yet
- Situational Analysis: Assessing Needs and Resources For VISION2020 Service Delivery and Identifying GAPSDocument11 pagesSituational Analysis: Assessing Needs and Resources For VISION2020 Service Delivery and Identifying GAPSPeter AbikoyeNo ratings yet
- OphthalmologyDocument6 pagesOphthalmologyPeter AbikoyeNo ratings yet
- Rich Text Editor FileDocument1 pageRich Text Editor FilePeter AbikoyeNo ratings yet
- Concept of PlanningDocument6 pagesConcept of PlanningPeter AbikoyeNo ratings yet
- GIT Essay QuestionsDocument2 pagesGIT Essay QuestionsPeter AbikoyeNo ratings yet
- Endocrinology Essay QuestionsDocument2 pagesEndocrinology Essay QuestionsPeter Abikoye0% (1)
- Snake Bite: Families of Venomous SnakesDocument4 pagesSnake Bite: Families of Venomous SnakesPeter AbikoyeNo ratings yet
- Technology Guidelines For A District Eye Care Programme: July 2006Document53 pagesTechnology Guidelines For A District Eye Care Programme: July 2006Peter AbikoyeNo ratings yet
- Management of Ocular BurnsDocument53 pagesManagement of Ocular BurnsPeter AbikoyeNo ratings yet