Professional Documents
Culture Documents
Purification, Peptide Sequencing, Modelling and Characterisation of Extracellular Laccase Secreted by Pleurotus Sajorcaju MTCC 141
Uploaded by
Dr. Antik BoseOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Purification, Peptide Sequencing, Modelling and Characterisation of Extracellular Laccase Secreted by Pleurotus Sajorcaju MTCC 141
Uploaded by
Dr. Antik BoseCopyright:
Available Formats
Purification, peptide sequencing, modelling and characterization of extracellular laccase secreted by Pleurotus sajor-caju MTCC 141
Antik Bose
Affilations I Contributions I Corresponding authors
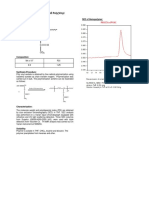
The extracellular laccase which is secreted by Pleurotus sajorcaju has been purified using Ammonium sulphate precipitation, DEAE-cellulose, Sephadex G-100, ConA-Sepharose 4B and HP1090A HPLC fitted with Vydac C-18 Reverse- Phase chromatography with 722.2 fold of purification and yield of 1.64%. 12% SDS-PAGE showed a monomer of 90KD. Western Blotting was done with monoclonal Lcc Cbr2 rabbit antibody raised against copper binding region II of laccase from Agaricus bisporus. Peptide sequencing of the purified laccase showed 529 amino acids long peptide with a 23 amino acids long signal peptide. Three Plastocyanin like domains were observed ( 25-159, 170-312 and 380-499). Residues 57, 239, 282 and 465 of the extracellular laccase showed Nlinked N-acetyl D-glucoseamine residues. The enzyme showed KM of 36M, 216pM, 380pM, 370pM and 260pM with 2,6 dimethoxyphenol, Catechol, m-cresol, pyrogallol and syringaldazine respectively. CuSo4, BaCl2, MgCl2, FeCL3, ZnCl3, Urea, PCMB, DTT, -mercaptoethanol had no effect on enzyme activity while HgCl2 and MnCl2 were weak inhibitors and SDS, Sodium azide were strong inhibitors. Crystallization data, collection, synchrotron radiation and processing have been carried out. Orthorhombic crystals of space group P2 12 121 w A, b= 85.0 A, c= 91.5 A 3/Da, assuming one molecule per asymmetric unit. Diffraction data was collected using Beamline BW7B of Synchroton Deutoches Elektonen Synchroton (DESY) at room temperature. Data was processed and scaled with programs DENZO and SCALEPACK of Hkl suit. For 1.9-20 A resolution shell, the data were 99.7% complete and scale with an overall Rsym of 0.063 and Rsym of 0.38 for data in highest resolution A resolution data, an Rcryst of 0.434 was obtained. A 2F 0Fe electron density map revealed clearly all four copper sites . Repeated rounds of refinement with REFMAC resulted in a complete and well-defined model with an Rwork A resolution. Only 4 out of total 499 amino acids were in generously allowed region of Ramachandran Plot.
You might also like
- LSM2191 Laboratory Techniques in Life SciencesDocument9 pagesLSM2191 Laboratory Techniques in Life SciencesAlun WinnNo ratings yet
- Isolation and Characterization of Pectin From Peel of Citrus TankanDocument4 pagesIsolation and Characterization of Pectin From Peel of Citrus TankanDaniel Landa LopezNo ratings yet
- Ijbt 6 (4) 549-552Document4 pagesIjbt 6 (4) 549-552anicica_866925No ratings yet
- NIHMS1850409 Supplement Supplementary - MaterialsDocument22 pagesNIHMS1850409 Supplement Supplementary - Materialsgundeepdsc8423No ratings yet
- Analytical ChemistryDocument7 pagesAnalytical ChemistryNia RukmanNo ratings yet
- Journal of Virology-1974-Chi-1194.fullDocument6 pagesJournal of Virology-1974-Chi-1194.fullshaeyhameedNo ratings yet
- Tailored Synthesis of Intelligent Polymer Nanocapsules: An Investigation of Controlled Permeability and pH-Dependant DegradabilityDocument22 pagesTailored Synthesis of Intelligent Polymer Nanocapsules: An Investigation of Controlled Permeability and pH-Dependant DegradabilityNeils ArenósNo ratings yet
- Denaturasi DnaDocument8 pagesDenaturasi DnadetiNo ratings yet
- Journal of Chromatographic PDFDocument8 pagesJournal of Chromatographic PDFGustavo Balarezo InumaNo ratings yet
- DNA biomolecular encoders and decoders constructed by multiplex biosensorsDocument6 pagesDNA biomolecular encoders and decoders constructed by multiplex biosensorsDavid PeñaNo ratings yet
- Deuterated Poly(Vinyl Acetate)-d6 SEC and CharacterizationDocument1 pageDeuterated Poly(Vinyl Acetate)-d6 SEC and Characterization8612106535No ratings yet
- The Enzymatic Activity of Proteinase K Is Controlled by CalciumDocument7 pagesThe Enzymatic Activity of Proteinase K Is Controlled by CalciumHaekal HafizhNo ratings yet
- Ag2 and Ag3 Clusters Synthesis, Characterization, and Interaction WithDocument16 pagesAg2 and Ag3 Clusters Synthesis, Characterization, and Interaction WithMariano PerezNo ratings yet
- An 'Equalized cDNA Library' by The Reassociation of Short Double-Stranded cDNAsDocument6 pagesAn 'Equalized cDNA Library' by The Reassociation of Short Double-Stranded cDNAsRoseNo ratings yet
- Determination of Acid Dissociation ConstDocument7 pagesDetermination of Acid Dissociation ConstDickson NaiNo ratings yet
- A Convenient Route to Selectively Protected Six-Carbon SynthonsDocument4 pagesA Convenient Route to Selectively Protected Six-Carbon SynthonsLuthfi RomdoniNo ratings yet
- CDK2 RoscovitineDocument9 pagesCDK2 RoscovitineputriNo ratings yet
- Csta20120300003 96506554Document5 pagesCsta20120300003 96506554Jeans Wills Ramirez RamirezNo ratings yet
- Marguerite Rinaudo: in GreatDocument10 pagesMarguerite Rinaudo: in GreatRabu ANo ratings yet
- High-Performance Liquid Chromatographic Separation and Chiroptical Properties of The Enantiomers of Naringenin and Other FlavanonesDocument8 pagesHigh-Performance Liquid Chromatographic Separation and Chiroptical Properties of The Enantiomers of Naringenin and Other Flavanonesfernando gonzalezNo ratings yet
- Solid State NMR For Determination of Degree of Acetylation ofDocument6 pagesSolid State NMR For Determination of Degree of Acetylation ofيحيى بورغدةNo ratings yet
- Synthesis of New Acridine-9-Carboxylic Acid DerivativesDocument6 pagesSynthesis of New Acridine-9-Carboxylic Acid DerivativesArévaloNo ratings yet
- Resolution of HydroxytamoxifenDocument6 pagesResolution of HydroxytamoxifenHari ShyamNo ratings yet
- Anion Exchange ChromatographyDocument5 pagesAnion Exchange ChromatographyIzzat EmranNo ratings yet
- Ye 2000Document8 pagesYe 2000h.sinner671No ratings yet
- Functional Electrospun Fibrous Scaffolds With Dextran-G-Poly (L-Lysine) - Vapg/Microrna-145 To Specially Modulate Vascular SmcsDocument9 pagesFunctional Electrospun Fibrous Scaffolds With Dextran-G-Poly (L-Lysine) - Vapg/Microrna-145 To Specially Modulate Vascular Smcssyedamasoomazahra9No ratings yet
- The Biocatalyzed Stereoselective Preparation of Polycyclic CyanohydrinsDocument7 pagesThe Biocatalyzed Stereoselective Preparation of Polycyclic CyanohydrinsDaigo G MamaniNo ratings yet
- Three-Dimensional Structure of P-GlycoproteinDocument6 pagesThree-Dimensional Structure of P-GlycoproteinSakshi IssarNo ratings yet
- Yumi Song, Chiyoung Park and Chulhee Kim - Synthesis and Self-Organization Characteristics of Amide Dendrons With Focal Ferrocenyl MoietyDocument5 pagesYumi Song, Chiyoung Park and Chulhee Kim - Synthesis and Self-Organization Characteristics of Amide Dendrons With Focal Ferrocenyl MoietyDremHpNo ratings yet
- Polylactide - Viscosity RelationshipsDocument7 pagesPolylactide - Viscosity RelationshipsQasim RiazNo ratings yet
- Supporting Information: Yong Yan, Ning-Ning Zhang, Lisa Marie Tauche, Andreas Pöppl, Harald KrautscheidDocument12 pagesSupporting Information: Yong Yan, Ning-Ning Zhang, Lisa Marie Tauche, Andreas Pöppl, Harald KrautscheidQuenton CompasNo ratings yet
- Kathrin S. COPLEY, Sheri M. ALM, David A. SCHOOLEY and William E. COURCHESNEDocument8 pagesKathrin S. COPLEY, Sheri M. ALM, David A. SCHOOLEY and William E. COURCHESNEBabbooNo ratings yet
- cs9b01900 Si 001Document119 pagescs9b01900 Si 001Duc AnhNo ratings yet
- HTTP WWW - Arkat-UsaDocument9 pagesHTTP WWW - Arkat-UsaGhayoor AbbasNo ratings yet
- 1992-A Novel Method For Isolation of Large Insert DNA FromDocument1 page1992-A Novel Method For Isolation of Large Insert DNA Fromrasime.demirelNo ratings yet
- Pnas00069 0078Document4 pagesPnas00069 0078Ashutosh SahuNo ratings yet
- Structures Results and Discussion: Xenobiotic Laboratories, Inc., 107 Morgan Lane, Plainsboro, NJ 08536Document1 pageStructures Results and Discussion: Xenobiotic Laboratories, Inc., 107 Morgan Lane, Plainsboro, NJ 08536Herry PrasetyantoNo ratings yet
- Biochimica EtbiophysicaactaDocument7 pagesBiochimica EtbiophysicaactaDemetriosNo ratings yet
- Applications CDDocument48 pagesApplications CDLiv IabNo ratings yet
- Determination of Lead and Copper in Wine by ASV-Sample Pretreatment ProceduresDocument5 pagesDetermination of Lead and Copper in Wine by ASV-Sample Pretreatment Procedurespaul_swiftNo ratings yet
- Highly Sensitive DNA Detection Using Aptamer-Modified Gold NanoparticlesDocument10 pagesHighly Sensitive DNA Detection Using Aptamer-Modified Gold NanoparticlesJohnnie LópezNo ratings yet
- Harzialactone TA 2005 LuDocument3 pagesHarzialactone TA 2005 LuhloicNo ratings yet
- jz2c01228 Si 001Document31 pagesjz2c01228 Si 001Kakali deviNo ratings yet
- Extraction of Cs and SR From Alkaline Solutions With High Nano Content With TetrahexyldicarbollideDocument23 pagesExtraction of Cs and SR From Alkaline Solutions With High Nano Content With TetrahexyldicarbollideHenry DelarueNo ratings yet
- Oger 2001Document8 pagesOger 2001FABIO ESTEBAN HERRERA ROCHANo ratings yet
- Angela Seidl and Hans-Jurgen Hinz - The Free Energy of DNA Supercoiling Is Enthalpy-DeterminedDocument5 pagesAngela Seidl and Hans-Jurgen Hinz - The Free Energy of DNA Supercoiling Is Enthalpy-DeterminedDopameNo ratings yet
- Electron Transfer and Paramagnetic Products of Reactions of Haloquinones With Aliphatic AminesDocument5 pagesElectron Transfer and Paramagnetic Products of Reactions of Haloquinones With Aliphatic AminesÖmür GezginNo ratings yet
- Enantioselective Reduction of Tetralones Using Fungal CulturesDocument6 pagesEnantioselective Reduction of Tetralones Using Fungal CulturesGabriella GabyNo ratings yet
- FulltextDocument7 pagesFulltextfnmendoncaNo ratings yet
- benzodiazepain like actionDocument6 pagesbenzodiazepain like actionMohamed KhedrNo ratings yet
- 21SP4920 12 25 18Document12 pages21SP4920 12 25 18azeNo ratings yet
- Delos 1982Document17 pagesDelos 1982asim shahzadNo ratings yet
- Crystal structure of the long-chain fatty acid transporter FadLDocument13 pagesCrystal structure of the long-chain fatty acid transporter FadLAaron PrideNo ratings yet
- Electronic Supplementary Material (ESI) For Organic & Biomolecular Chemistry. This Journal Is © The Royal Society of Chemistry 2022Document239 pagesElectronic Supplementary Material (ESI) For Organic & Biomolecular Chemistry. This Journal Is © The Royal Society of Chemistry 2022Sofia bbNo ratings yet
- Compuestos de CoordinaciónDocument2 pagesCompuestos de CoordinaciónnathaloaNo ratings yet
- Determination of Disinfectant Imagard ID-401 Residue Post Application On SurfaceDocument7 pagesDetermination of Disinfectant Imagard ID-401 Residue Post Application On SurfaceSurjeet SamantaNo ratings yet
- DC Electrical Conductivity of Iodine Doped Tetrapyrazino Vanadyl OxideDocument6 pagesDC Electrical Conductivity of Iodine Doped Tetrapyrazino Vanadyl OxideTJPRC PublicationsNo ratings yet
- Gel Electrophoresis of ProteinsFrom EverandGel Electrophoresis of ProteinsMichael J DunnNo ratings yet
- Electrochemical Processes in Biological SystemsFrom EverandElectrochemical Processes in Biological SystemsAndrzej LewenstamNo ratings yet
- Application of IC-MS and IC-ICP-MS in Environmental ResearchFrom EverandApplication of IC-MS and IC-ICP-MS in Environmental ResearchRajmund MichalskiNo ratings yet
- TMP E572Document6 pagesTMP E572FrontiersNo ratings yet
- Acanthosis Nigricansin PCOS Patients and Its Relation With Type 2 Diabetes Mellitus and Body Mass at A Tertiary Care Hospital in Southern IndiaDocument3 pagesAcanthosis Nigricansin PCOS Patients and Its Relation With Type 2 Diabetes Mellitus and Body Mass at A Tertiary Care Hospital in Southern IndiaDr. Antik BoseNo ratings yet
- A Critical Appraisal of Methods To Grade Transplant Glomerulitis in Renal Allograft BiopsiesDocument11 pagesA Critical Appraisal of Methods To Grade Transplant Glomerulitis in Renal Allograft BiopsiesDr. Antik BoseNo ratings yet
- Impact of Genomic Sequence Variability On Quantitative PCR Assays For Diagnosis of Polyomavirus BK InfectionDocument6 pagesImpact of Genomic Sequence Variability On Quantitative PCR Assays For Diagnosis of Polyomavirus BK InfectionDr. Antik BoseNo ratings yet
- Alemtuzumab Pre-Conditioning With Tacrolimus Monotherapy in Pediatric Renal TransplantationDocument7 pagesAlemtuzumab Pre-Conditioning With Tacrolimus Monotherapy in Pediatric Renal TransplantationDr. Antik BoseNo ratings yet
- Tomatoes Versus Lycopene in Oxidative Stress and Carcinogenesis: Conclusions From Clinical TrialsDocument11 pagesTomatoes Versus Lycopene in Oxidative Stress and Carcinogenesis: Conclusions From Clinical TrialsDr. Antik BoseNo ratings yet
- Alemtuzumab Pre-Conditioning With Tacrolimus Monotherapy in Pediatric Renal TransplantationDocument7 pagesAlemtuzumab Pre-Conditioning With Tacrolimus Monotherapy in Pediatric Renal TransplantationDr. Antik BoseNo ratings yet
- Cellular and Physiological Mechanisms of New-Onset Diabetes Mellitus After Solid Organ TransplantationDocument12 pagesCellular and Physiological Mechanisms of New-Onset Diabetes Mellitus After Solid Organ TransplantationDr. Antik BoseNo ratings yet
- The Penumbra System: A Mechanical Device For The Treatment of Acute Stroke Due To ThromboembolismDocument5 pagesThe Penumbra System: A Mechanical Device For The Treatment of Acute Stroke Due To ThromboembolismDr. Antik BoseNo ratings yet
- Transplantation of en Bloc Kidneys From Very Small Pediatric DonorsDocument1 pageTransplantation of en Bloc Kidneys From Very Small Pediatric DonorsDr. Antik BoseNo ratings yet
- Adjuvant Effect and TLR4 Mediated Activation of Macrophages by Polysaccharide From Polyporus AlbicansDocument21 pagesAdjuvant Effect and TLR4 Mediated Activation of Macrophages by Polysaccharide From Polyporus AlbicansDr. Antik BoseNo ratings yet
- Purification and Characterization of Isozyme 1 and 2 of Acid Phosphatase From Cicer ArietinumDocument2 pagesPurification and Characterization of Isozyme 1 and 2 of Acid Phosphatase From Cicer ArietinumDr. Antik BoseNo ratings yet
- A Comparison of Physiological & Biochemical Changes of Ricinus Communis L. Leaf in Fresh and Polluted Environment.Document2 pagesA Comparison of Physiological & Biochemical Changes of Ricinus Communis L. Leaf in Fresh and Polluted Environment.Dr. Antik BoseNo ratings yet
- Chronic Allograft Nephropathy Score Before Sirolimus Rescue Predicts Allograft Function in Renal Transplant PatientsDocument10 pagesChronic Allograft Nephropathy Score Before Sirolimus Rescue Predicts Allograft Function in Renal Transplant PatientsDr. Antik BoseNo ratings yet
- Purification and Characterization of Alkaline Phosphatse From Pleurotus Sajor-CajuDocument2 pagesPurification and Characterization of Alkaline Phosphatse From Pleurotus Sajor-CajuDr. Antik BoseNo ratings yet
- Purification of Urease From Pleurotus Sajor-Caju. Characterization of GTPase Activity of UreG and Biochemical Properties of The EnzymeDocument1 pagePurification of Urease From Pleurotus Sajor-Caju. Characterization of GTPase Activity of UreG and Biochemical Properties of The EnzymeDr. Antik BoseNo ratings yet
- Purification, Peptide Sequencing and Study of Anti-Proliferative Activity of Laccase Against Liver Cancer Cell Line HepG2 and Human Breast Cancer Cell Line MCF7Document1 pagePurification, Peptide Sequencing and Study of Anti-Proliferative Activity of Laccase Against Liver Cancer Cell Line HepG2 and Human Breast Cancer Cell Line MCF7Dr. Antik BoseNo ratings yet
- Purification and Characterisation of Alkaline Phosphatase From Fruticose Lichen UsneaDocument2 pagesPurification and Characterisation of Alkaline Phosphatase From Fruticose Lichen UsneaDr. Antik BoseNo ratings yet
- Adjuvant Effect and TLR-4 Mediated Activation of Macrophages by Polysaccharide From Polyporus AlbicansDocument2 pagesAdjuvant Effect and TLR-4 Mediated Activation of Macrophages by Polysaccharide From Polyporus AlbicansDr. Antik BoseNo ratings yet
- Purification, Peptide Sequencing and Modelling of Ostreolysin From Pleurotus Ostreatus Strain Plo5. Formation of A Modified Ostreolysin With Anticancer Activity Against Cancer Cell LinesDocument23 pagesPurification, Peptide Sequencing and Modelling of Ostreolysin From Pleurotus Ostreatus Strain Plo5. Formation of A Modified Ostreolysin With Anticancer Activity Against Cancer Cell LinesDr. Antik BoseNo ratings yet