Professional Documents
Culture Documents
Function and Evolution of The Frill of The Frillneck Lizard, Chlamydosaurus Kingii (Sauria: Agamidae)
Function and Evolution of The Frill of The Frillneck Lizard, Chlamydosaurus Kingii (Sauria: Agamidae)
Uploaded by
Abdilatif MohamudOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Function and Evolution of The Frill of The Frillneck Lizard, Chlamydosaurus Kingii (Sauria: Agamidae)
Function and Evolution of The Frill of The Frillneck Lizard, Chlamydosaurus Kingii (Sauria: Agamidae)
Uploaded by
Abdilatif MohamudCopyright:
Available Formats
Biological Journal of the Linnean Society (1990), 40: 11-20.
With 2 figures
Function and evolution of the frill of the frillneck lizard, Chlamydosaurus kingii (Sauria:
Agamidae)
RICHARD SHINE
<oology Department, The University o f Sydney, N.S.W. 2006, Australia
Received 23 January 1989, accepted f o r publication 26 M a y 1989
Relative to body size, the frill of the Australian agamid lizard Chlamydosaurus kingii is one of the largest and most spectacular display structures seen in any animal species. More than 300 hours observation of free-ranging lizards, combined with data on museum specimens, revealed that the frill is used primarily for intraspecific communication and predator deterrence. Earlier hypotheses on alternative uses for the frill (gliding, food storage, thermoregulation or auditory enhancement) are not supported. The folded frill may also enhance camouflage, but this is probably a fortuitous effect rather than an adaptation. Male frillnecks frequently display and fight during the mating season. Male displays are highly stereotyped, and involve repeated partial erection of the frill, head-bobbing, tail-lashing, and waving of forelimbs. Both males and females erect the frill during social encounters, and in response to potential predators. Males grow larger than females and have larger heads than do females at the same body size, but no dimorphism is apparent in the relative size of the frill. The extreme development of the display structure in this species may be due to general allometric relationships, as well as to ecological features that have intensified the action of sexual selection in Chlamydosaurus. KEY WORDS:-Sauria
-
Agamidae
display - allometry - sexual dimorphism. CONTENTS
Introduction . . . Material and methods. Results . . . . Museum data. . Field data . . Discussion. . . . Acknowledgements . . . . References
. . . . . . . .
.
.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . .
INTRODUCTION
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
. . . . . .
. . . . . .
. . . . . .
. 11 . . 12 . 14 . . 14 . . 14 . 17 . . 19 . 19
Elaborate organs for display have evolved independently in many groups of animals, both invertebrate and vertebrate (Darwin, 187 1). The functional significance of such structures, and the selective pressures likely to have been important in their evolution, may be clarified by observations of the ecological context in which the display is performed, and by details of the morphology of the structure (e.g. Darwin, 1871; Harvey, Kavanagh & Clutton-Brock, 1978). However, caution is needed in extrapolating from current function to
0024-4066/90/050011+ 10 $03.00/0
11
0 1990 The Linnean Society of London
12
R. SHINE
evolutionary origin: a structure may have originally evolved for some reason other than its present function, or have arisen through general allometric relationships rather than specific selective pressures (Huxley, 1932; Gould & Lewontin, 1979). These issues may be clarified by the study of extreme elaborations of display structures, such as the renowned antlers of the Irish Elk (Gould, 1974). The present study examines the functional significance, and probable adaptive significance, of the largest and most dramatic display structure seen in any reptile: the frill of the Australian frillneck lizard, Chlamydosaurus kingii. This large (to 90 cm total length) agamid lizard of tropical Australia and southern New Guinea possesses a n enormous frill, a fold of skin attached to the neck, supported by hyoid cartilage and covered with large keeled scales. Ordinarily the frill is kept folded back against the body, but it can be erected to a diameter more than four times that of the lizards body (Fig. 1). Frill erection is commonly seen as a defensive response to human interference, but the lack of information on behaviour of Chlamydosaurus in the field means that other potential uses for the frill remain entirely speculative. The frill has been variously interpreted as an adaptation for gliding (Fenner, 1933), food storage (Bacchus, 1939), increasing auditory acuity (De Vis, 1883), thermoregulation (Worrell, 1963; Frith & Frith, 1987), predator deterrence (Kent, 1895), or courtship display (Daan, 1972). Other possibilities, such as camouflage or male territorial display, seem not to have been discussed.
MATERIAL AND METHODS
Body length, head size and frill size were measured on preserved specimens at the Australian Museum. Sex was determined by midventral incision and inspection of the gonads. Body length was measured from the tip of the snout to the vent, and head length from the tip of the snout to the posterior extremity of the lower jaw. Frill length was measured inside the frill, from the attachment point closest the end of the lower jaw to the furthest extremity of the frill in a line parallel to the main axis of the lizards body. Observations of free-ranging Chlamydosaurus kingii were carried out in Kakadu National Park, 250 km east of Darwin in Australias Northern Territory. Most work was centred on the Four-gates Road, which runs along the western side of Magela Creek downstream of Magela Crossing. Kakadu lies within the wetdry tropics, with high temperatures year-round (daily maxima at the nearest town, Jabiru, average 31C in July, 39C in October: Brennan, 1986), but with annual rainfall highly seasonal (over 90% in the months December to March). Chlamydosaurus is abundant in savannah woodland in this region, especially in the open forests dominated by Eucalyptus tetrodonta and E.miniata. Most of the information reported in the present paper comes from observation of 11 adult frillnecks in which miniature temperature-sensitive radiotransmitters were 1986. These data were surgically implanted in November-December supplemented by observations on other animals during the same period, and data from seven other telemetered lizards in other years. This work was part of a larger study on habitat use, food habits, movements and thermal relations of this species (Shine & Lambeck, 1989). Lizards were initially located by surveillance from a vehicle driving slowly through suitable habitat. The animals were captured by hand, and returned to
DISPLAY STRUCTURES
13
Figure 1 . Threat display by adult male frillneck lizard.
the laboratory. Small radiotransmitters (50 x 20 mm, 15 g; TT-IU-080, J. Stuart Enterprises) were surgically inserted in the peritoneal cavity under cold anaesthesia. The incisions were sutured closed, and all lizards were released within 2 4 h of the surgery, at the exact site of their original capture. The transmitters weighed from 2 to 6% of lizard body mass, and did not prevent subsequent movement, social interactions, foraging or oviposition (see below and
14
R. SHINE
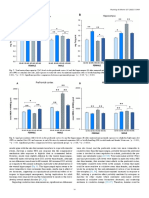
Snout-vent length (cm)
Figure 2. Measurements of preserved frillneck lizards reveal sexual dimorphism in jaw length relative to body length (left), but not in frill length relative to body length (right). See text for definitions. methods and statistics.
Shine & Lambeck, 1989). Effective reception range for the telemetry signal averaged 500 m. Lizard behaviour was monitored during daylight hours over the following three weeks. In the November-December 1986 study, observers sat approximately 20 m from the lizards in portable hessian hides (2 x 1 x 1 m), with binoculars inserted through a small slit cut in the front of the blind. A total of > 300 hours of observations was carried out; details of methodology and monitoring schedules are given in Shine & Lambeck (1989).
RESULTS
Museum data
Measurements of preserved Chlamydosaurus kingii showed that males attain much larger body sizes than do females (Northern Territory specimens only: mean male snout-vent length (SVL) = 254.2 mm, s.d. = 21.9, n = 56; mean female SVL=206.4, s.d.= 16.6, n = 16; t=8.08, 70d.f., P < O . O O l ) . Males also have larger heads than do females at the same body length (Fig. 2: analysis of covariance, slopes F1,21 = 3.32, n.s.; intercepts F1,22= 13.06, P<O.Ol), but frill length relative to body length does not differ between the sexes (slopes F1,21 =0.48, n.s., intercepts F1,22=0.01, n.s.). Frills are proportionately much smaller in smaller animals, so that the larger body sizes of male frillnecks mean that the ratio of frill length to body length is slightly higher in males (means of 0.30 us. 0.32, t = 0.76, 23 d.f., P= 0.46). However, the two sexes appear to follow the same allometric relationship in this respect (Fig. 2).
Field data
Data gathered on the ecological context of frill erection enabled evaluation of alternative hypotheses proposed for functions of the frill. (1) Gliding. Early reports of frillnecks using their erect frills as parachutes, to glide from tree to tree (Fenner, 1933) seem unlikely to be reliable. We observed frillnecks descending from the trees to forage on the ground on ten occasions, and often saw them moving among branches within a tree (sometimes jumping between branches up to 1.3 m apart). In none of these instances was there any
DISPLAY STRUCTURES
15
evidence of frill erection. O n a priori grounds, the frill seems too small and fragile to be significant as a parachute. (2) Food storage. The idea that frillnecks may store food items within the folds of their frill (Bacchus, 1939) has no empirical support from this study. Most lizards that we captured contained food items in their stomach, and some lizards were so full of prey that food items fell from their mouths when they gaped to display at us. None contained prey items within the frill and, as in the case of the previous hypothesis, it is difficult to imagine this phenomenon on a priori grounds. (3) Auditory enhancement. De Vis (1883: 301) speculated that one function of the hood might be that of conducting sound to the tympanum, an office apparently aided by the channels formed by its converging folds. His dissection of the frill gave no support to his hypothesis, and we did not observe frill erection in response to unusual auditory stimuli. Most cases of frill erection (see below) were clearly inconsistent with enhancement of auditory capability. (4) CamouJage. Although the frill is very obvious when erect, it is inconspicuous when at rest, folded back along the body (Fig. 1). The folds of the frill resemble loose shreds of bark or dry leaves, and to some extent break up the outline of the lizard. Although this enhancement of camouflage may be of advantage to the lizards, it is most parsimoniously interpreted as a secondary consequence of the frill rather a selective pressure for the evolution of this large erectile structure. ( 5 ) Thermoregulation. Because the erect frill has a large surface area, there is the potential for it to serve as a device for collecting radiant energy, dissipating excess heat to the air, or perhaps shading the lizards body to reduce exposure to the suns rays (Worrell, 1963; Frith & Frith, 1987). The first of these hypotheses predicts that frill erection should be seen when the lizard is basking, which typically occurs early in the morning (Shine & Lambeck, 1989). Frill erection was never observed in this context: basking lizards were able to elevate body temperatures rapidly without erecting the frill. In any case, it would be remarkable to see the evolution of a structure to facilitate heat uptake in a reptile living in this very warm climate: lizard temperatures average close to 40C (similar to ambient temperature) in the mid-afternoon (Shine & Lambeck, 1989). The highly seasonal activity patterns of Chlamydosaurus also mean that it is rarely active in cooler times of the year (Shine & Lambeck, 1989). Overheating may be a more significant problem, but we saw no evidence of frill erection for this purpose even on very hot days. Experiments in which the frill was surgically removed (and subsequently taped back in place) suggested that it had little or no effect on the rate at which body temperatures increased in tethered Chlamydosaurus exposed to a heat source (G.J. W. Webb, personal communication). (6) Predator deterrence. This is the function most often attributed to the frill. All lizards except one juvenile erected the frill when they were captured, and also whenever we handled them in captivity. It seems likely that similar responses are given to naturally occurring predators, such as hawks (D. Curl, personal communication), varanid lizards (Shine, 1986), and elapid and boid snakes (Shine, unpublished observations). We observed partial frill erection by lizards in response to the close approach of birds (orioles, kookaburras). One lizard displayed full frill erection in response to a motor vehicle travelling approximately 50 m away.
16
R.SHINE
(7) Social interactions. Many agamid lizards show complex social behaviour, including assertion displays by territorial adult males. Within the Australian Agamidae, such displays have been reported in the genera Amphibolurus, Ctenophorus and Pogona (e.g. Carpenter, Badham & Kimble, 1970; Greer, 1988). In the bearded dragon (Pogona) the beard is used in social contexts as well as in predator deterrence (Carpenter et al., 1970). It is thus surprising that little attention seems to have been given to the potential role of the Chlamydosaurus frill in social interactions. O u r observations suggest that intraspecific communication is the primary function of the frill. T h e most common contexts in which we saw frill erection in free-ranging lizards were (a) displays by adult male lizards, and (b) interactions between lizards. (a) Male displays. All adult males ( n = 5 telemetered, 8 non-telemetered) observed in November-December 1986 engaged in frequent and spectacular displays. These displays were not directed to other specific individuals. Displays were seen throughout the day, but with a higher incidence in the early morning: 33% of all records were obtained between 0600 and 0800 hours, and only 16% in the much longer period between 1100 and 1800 hours. Thus, these displays may be broadly comparable to the non-directed territorial defence seen in the dawn chorus of many bird species. Of 21 display sequences, lasting from a few minutes to > 1 hour, five were on the ground, rocks or small fallen logs whereas the remainder were in trees. Most commonly (14 of 16 arboreal cases), males showed this behaviour while they were clinging, head upwards, to relatively slender vertical trunks of standing trees. In the other cases, males were headdownwards during the. display. The heights of trees used for displays (mean= 12.7 m, s.d.=3.67, n = 17) were significantly greater than the mean height of 341 trees measured in transects through the study area (mean = 8.3 m, t=4.88, 16d.f., P<0.002: see Shine & Lambeck, 1989, for sampling details). However, no selection was apparent with respect to the species of trees used for displays: we recorded this behaviour on eight of the most common tree species, in proportions that were not significantly different from those in our transects (contingency x2=5.66, 7d.f., P=0.58) or in comparison to all trees used by telemetered frillnecks (x2= 1 1.21, 7 d.f., P= 0.13). The diameter of the tree-trunk on which the display was performed averaged 17.7 cm (n = 72, s.d. = 13.24), and ranged from 10 to 60 cm. In many cases, the trunk was sufficiently narrow that the frill erection could be clearly seen even if the observer was on the other side of the tree from the lizard. Arboreal displays were performed a n average of 4.29 m above the ground, with a range of 1 to 10 m. The display was invariant in general form, but variable in duration and in some details (e.g. mouth-gaping and limb-waving were not noted in some sequences). The general form of the display was as follows. First, the lizard lashed its tail vigorously from side-to-side (act system No. 93 of Carpenter & Ferguson, 1977) against the bark of the tree a few times (mean=3.2 lashes, range 2 to 4),resulting in a sound that was clearly audible to observers > 30 m distant. Th e lizard then raised the forepart of its body slightly from the treetrunk by means of simultaneous extension of both forelimbs (as in a push-up display: act system No. 73 of Carpenter & Ferguson, 1977). This was followed immediately by a series of partial frill erections (generally, one-third to one-half of full erection) with simultaneous head-bobbing and slight opening of the mouth (act systems No. 75 and 6). Each display sequence comprisedan average
DISPLAY STRUCTURES
17
of 9.25 frill erections (s.d. =6.77, n=57, range= 1 to 29), and lasted an average of 11.3 seconds (range 5 to 30). The lizard would then wait for 1 to 40 minutes (n = 72, mean = 7.3 minutes, s.d. = 8.10) before commencing another display. In about one-third of displays, limb circumdiction (waving of one foreleg) was also evident. Frill erections were usually, but not always, simultaneous with headbobs and gaping. These assertion displays were generally associated with relocation of the lizard, with males often moving up, down or around the trunk between successive displays. Less frequently, displaying males left their tree and moved bipedally across the ground, reverting to a quadrupedal posture to display. Often, such displays were performed on rocks or logs, or at the base of other trees (sometimes, clinging approximately 1 m up the trunk). However, the time spent on the ground rarely exceeded a few minutes. (b) Male-male interactions. Frillnecks mate in November-December, and battles between males are common. Males fight by lunging at each other head-on and interlocking their jaws (observed on 19 November 1986). Mouths are opened widely in displays prior to combat, and the frill is fully erected. As a consequence of this mode of fighting, injuries to the jaws are common in adult males during the breeding season. Of 29 adult males examined during November-December 1986, 26 (90%) had obvious scarring, mostly recent (seven with fresh blood evident on the wounds). Of these 26 animals, injuries to the jaws were seen in 18 (69%), ranging from missing teeth to actual breaks in the mandible. In contrast, scarring was recorded in only one of four immature males (25%), and one of eight adult females (12.5%). These data reveal a significantly higher incidence of injuries in adult males than in either females (x2=20.6, 1 d.f., P<O.OOl) or juvenile males (x2= 12.66, 1 d.f., P<O.OOl). Remarkably, males continued to feed even with freshly broken lower jaws (as judged by plentiful prey items in stomachs of such animals). (c) Male-female interactions. O n two occasions when male and female lizards occupied the same tree, partial frill erection and head-bobbing were shown by both sexes. Mating was not observed. Solitary female lizards did not engage in displays, nor did a juvenile male monitored at the same time, nor four adult lizards (including one female) monitored for 1 month in April 1986 after the conclusion of the mating season.
DISCUSSION
Th e frill of Chlamydosaurus is an extreme elaboration of a virtually universal trait among agamid lizards: an erectile throat fan, supported by the hyoid apparatus (e.g. Moody, 1980). The primary function of such ornaments seems usually to be for intraspecific communication, and especially for assertion displays by territorial males (e.g. Carpenter & Ferguson, 1977; Greer, 1988). Data presented above show that Chlamydosaurus conforms to these general patterns. Although the frill is much larger than equivalent structures of other reptiles, it is used as a device for social signalling in the same way as smaller structures are used in other agamid lizards. Chlamydosaurus also resembles its relatives in using the frill for deterring potential predators. Other suggested functions of the frill seem unlikely to be valid. In particular, the behavioural data strongly falsify predictions from hypotheses that the frill is used for volplaning, food storage or auditory enhancement. The frill also seems unlikely
18
R. SHINE
to have any thermoregulatory significance, and its contribution to camouflage is more likely to be a secondary consequence of frill morphology than a selective pressure for the evolution of the structure. Both of the remaining hypotheses-anti-predation and intraspecific display-are supported by our data. By far the commonest use of the frill, at least during the mating season, is in displays by males and in interactions between adult lizards. The apparent lack of frill displays by solitary females and juvenile males suggests, by analogy with studies on other agamids (e.g. Carpenter & Ferguson, 1977), that the male display functions in territorial defence. Structures used by males in this way often show strong sexual dimorphism (e.g. antlers and tusks in male ruminants), but relative frill size is not dimorphic in C. kingii (Fig. 2). The dimorphism documented in relative head sizes of this species is similar to that reported in many other lizards, in which it has been attributed to sexual selection for success in male-male combat (e.g. Carothers, 1984; Vitt & Cooper, 1986). The lack of dimorphism in frill size can be explained in at least two ways. (i) Females also use the frill for intraspecific communication and deterrence of predators (and perhaps for other purposes). Unless there is some selective disadvantage to large frill size in females, sexual selection for large frills in males would also increase the size of the structure in females (Fisher, 1930). (ii) Even if large frill size is disadvantageous to females, high genetic correlation between the sexes may prevent the evolution of sexual dimorphism (Lande, 1980). This hypothesis is difficult to reconcile with the observed dimorphism in relative head sizes. The obvious question remaining is: why is the display structure of this species so much larger, relative to body size, than in any other reptile (and virtually any other animal)? The dramatic assertion display of male Chlarnydosaurus, and the elaboration of the frill for that display, are extreme developments of tendencies that are widespread in other lizard species. The combat between rival males, and the consequent serious injuries (especially, mandible breakage in adult males during the breeding season) are also more exaggerated than the situation in most other lizards. Why should frillnecks be extreme in these respects? Several factors may increase the potential variance in reproductive success among males and hence, the opportunities for sexual selection. Sexual competition between males is likely to be most intense when a single male can potentially inseminate several females. Such a situation may arise from: (i) the relatively extended season over which receptive females may become available (as judged by the timing of oviposition of captured gravid females, and the seasonal distribution of such females: Shine & Lambeck, 1989); (ii) the high abundances of this species in suitable habitats; (iii) the open nature of the habitat, coupled with the high visual acuity of the lizards, so that lizards can interact with conspecifics over a wide area; and (iv) the high mobility of individuals of this species (Shine & Lambeck, 1989). These characteristics may increase the variance in male reproductive success and the intensity of sexual selection. This in turn may facilitate the extreme development of sexually-selected morphology and bahaviour. An alternative hypothesis for the extreme elaboration of the frill involves the concept of allometry. Frills are small in hatchlings, and relative frill size increases with body size (e.g. Fig. 2). Similar positive allometry of display structures occurs both intraspecifically and interspecifically in many other types of animals
DISPLAY STRUCTURES
19
(e.g. Gould, 1974), including other agamid lizards (Moody, 1980). Hence, the large relative frill size in Chlamydosaurus may result simply from the unusually large body size of this species. Body size is much larger in Chlamydosaurus than in any related genus (e.g. Lophognuthus: Moody, 1980). Given the putatively short time-span of the Australian agamid radiation (based on molecular data: P. Baverstock, personal communication), average body size may have increased relatively rapidly in the lineage leading to Chlamydosaurus. If so, the relative size of the frill may have similarly increased through adherence to this general positive allometry, rather than through specific selective pressures for an unusually large display structure. The evolution of the spectacular frill in Chlamydosaurus kingii may reflect both allometry and adaptation. Given that these are not mutually exclusive alternatives, it may be impossible to test between the two hypotheses.
ACKNOWLEDGEMENTS
Field assistance was provided by R. Lambeck, T. Pople, T. Press, R. Hore and D. Houston. Logistical support came from Pancontinental Mining Co., the Office of the Supervising Scientist, and the Australian National Parks and Wildlife Service. G. J. W. Webb and D. Curl provided unpublished data, and T. Pople, C. James and D. Houston provided comments on the manuscript. The study was funded by the Australian Geographic Scientific Research and Exploration Fund.
REFERENCES BACCHUS, J., 1939. Notable Australian lizards. Walkabout, 5: 53-60. BRENNAN, K., 1986. Wildflowers of Kakadu. Jabiru, N. T.: Privately published. CAROTHERS, J. H., 1984. Sexual selection and sexual dimorphism in some herbivorous lizards. American Naturalist, 124: 244-254. CARPENTER, C. C. & FERGUSON, G. W., 1977. Variation and evolution of sterotyped behavior in reptiles. In C. Gans & D. W. Tinkle (Eds), Biology o f t h e Reptilia, 7: 335-554. New York: Academic Press. CARPENTER, C. C., BADHAM, J. A. & KIMBLE, B., 1970. Behavior patterns of three species of Amphibolurus (Agamidae). Copcia, 1970: 497-505. D U N , S., 1972. Family Agamidae. I n B. Grzimek (Ed.), Animal L q e Encyclopedia: 205-227. New York: van Nostrand Reinhold Co. DARWIN, C., 1871. The Descent o f M a n and Selection in Relation to Sex. London: Murray. f the Linnean Society o f N e w South Wales, I : DE VIS, C. W., 1883. Myology of Chlamydosaurus kingii. Proceedings o 300-320. FENNER, C., 1933. Runyips and billabongs. Sydney: Angus & Robertson. f Natural Selection. Oxford: Oxford University Press. FISHER, R. A,, 1930. The Genetical Theory o FRITH, C. & FRITH, D., 1987. Australian Tropical Reptiles and Frogs. Townsville: Tropical Australia Graphics. GOULD, S. J., 1974. The origin and function of 'bizarre' structures: antler size and skull size in the "Irish Elk", Megaloceros giganteus. Euolution, 28: 191-220. GOULD, S. J. & LEWONTIN, R. C., 1979. The spandrels of San Marco and the panglossian paradigm: A critique of the adaptationist programme. Proceedings o f the Royal Society of London, R205: 581-598. GREER, A. E., 1988. Australian Lizards. Sydney: Surrey Beatty & Co. HARVEY, P. H., KAVANAGH, M. J. & CLU ON-BROCK, T. H., 1978. Sexual dimorphism in primate teeth. Journal of ~ o o l o g y ,186: 475486. HUXLEY, J. S., 1932. Problems of Relatiue Growlh. London: Methuen. KENT, W. S., 1895. Observations on the frilled lizard, Chlamydosaurus kingi. Proceedings of the <oological Society, 1895: 712-719. LANDE, R., 1980. Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Euolution, 34: 292-305. MOODY, S. M . , 1980. Phylogenetic and historical biogeographical relationships o f the genera in the f a m i b Agamidae (Reptilia: Lacertilia). Unpublished doctoral thesis, University of Michigan, Ann Arbor, Michigan.
20
R. SHINE
SHINE, R., 1986. Food habits, habitats and reproductive biology of four species of varanid lizards in tropical Australia. Herpetologica, 42: 346-360. SHINE, R. & LAMBECK, R., 1990. Ecology of frillneck lizards, Chlzmydosaurus kingii (Agamidae) in tropical Australia. Australian Wild& Research, in press. VITT, L. J. & COOPER, W. E., 1986. Skink reproduction and sexual dimorphism: Eumeces farciatus in the f Herpetology, 20: 4084.15. southeastern United States, with notes on Eumeces inexpectatus. Journal o WORRELL, E., 1963. Reptiles o f Australia. Sydney: Angus & Robertson.
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5819)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1092)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (845)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (540)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (348)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (822)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- MRR1 Ged 109 A5-MalabananDocument2 pagesMRR1 Ged 109 A5-MalabananRose Anne Malabanan67% (3)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Dustin R. Rubenstein, John Alcock - Animal Behavior-Sinauer Associates Is An Imprint of Oxford University Press (2018)Document667 pagesDustin R. Rubenstein, John Alcock - Animal Behavior-Sinauer Associates Is An Imprint of Oxford University Press (2018)João Pedro Magalhaes100% (2)
- Bucolingual Dimension of TeethDocument4 pagesBucolingual Dimension of TeethVicky Rahmaniah SyamsuryaNo ratings yet
- Men and Women Show Distinct Brain Activations During Imagery of Sexual and Emotional InfidelityDocument9 pagesMen and Women Show Distinct Brain Activations During Imagery of Sexual and Emotional InfidelityGiulio Annicchiarico LoboNo ratings yet
- Unit 3 Behavioral EcologyDocument27 pagesUnit 3 Behavioral EcologyVenus AnteroNo ratings yet
- The Raw and The StolenDocument28 pagesThe Raw and The StolendanielarssNo ratings yet
- Sexual Dimorphism in Human BodyDocument9 pagesSexual Dimorphism in Human BodyAlejandro VegaNo ratings yet
- Sex and Gender ReportDocument30 pagesSex and Gender ReportFerlynne Marie BernardinoNo ratings yet
- Blue Tits Are Ultraviolet Tits: Sarah Hunt, Andrew T. D. Bennett, Innes C. Cuthill and Richard Gri ThsDocument6 pagesBlue Tits Are Ultraviolet Tits: Sarah Hunt, Andrew T. D. Bennett, Innes C. Cuthill and Richard Gri Thsrehman khanNo ratings yet
- Evolutionary Psychology and Feminism - Final Published 2011Document20 pagesEvolutionary Psychology and Feminism - Final Published 2011Rodrigo Caetano RahmeierNo ratings yet
- Lassek & Gaulin - Muscle Mass IMPDocument7 pagesLassek & Gaulin - Muscle Mass IMPPsic Israel Juárez RodríguezNo ratings yet
- Sex Differences in Brain and Behavior in Adolescence Gur 2016Document12 pagesSex Differences in Brain and Behavior in Adolescence Gur 2016Andra-Amalia ArmulescuNo ratings yet
- Framed by Gender: Cecilia L. RidgewayDocument20 pagesFramed by Gender: Cecilia L. Ridgewayyoni hathahanNo ratings yet
- The Socio-Sexual Behaviour of Extant Archosaurs - Implications For Understanding Dinosaur BehaviourDocument77 pagesThe Socio-Sexual Behaviour of Extant Archosaurs - Implications For Understanding Dinosaur BehaviournomadNo ratings yet
- (2018) Sex and Gender Differences Research Design For Basic, Clinical, and Population Studies - Essentials For InvestigatorsDocument16 pages(2018) Sex and Gender Differences Research Design For Basic, Clinical, and Population Studies - Essentials For InvestigatorsXavierNo ratings yet
- Sexual Shape Dimorphism in Monomorphic Fish Decapterus Macrosoma PDFDocument9 pagesSexual Shape Dimorphism in Monomorphic Fish Decapterus Macrosoma PDFKaent Immanuel UbaNo ratings yet
- The Wandering Eyes of Men and Women - Sex Differences in Motivations For InfidelityDocument20 pagesThe Wandering Eyes of Men and Women - Sex Differences in Motivations For InfidelityQuel PaivaNo ratings yet
- 3.6.induced Breeding of Indian Major CarpsDocument14 pages3.6.induced Breeding of Indian Major CarpsAlivia Roy100% (1)
- Sexual Dimorphism in Morphological Traits and Food Habits of Rana TigrinaDocument10 pagesSexual Dimorphism in Morphological Traits and Food Habits of Rana TigrinaRyan Carlo CondeNo ratings yet
- Sexual Selection: How Does It WorkDocument7 pagesSexual Selection: How Does It WorkRizwana MiduryNo ratings yet
- Life History and Morphometry of The Chinese Praying MantisDocument13 pagesLife History and Morphometry of The Chinese Praying MantisJulian Andres Rodriguez PovedaNo ratings yet
- EaglyWoodvanLange CH 49Document20 pagesEaglyWoodvanLange CH 49بشرىNo ratings yet
- Sex Differs in BrainDocument14 pagesSex Differs in BrainSex & Gender Women's Health CollaborativeNo ratings yet
- The Evolutionary Psychology of Men's Coercive SexualityDocument59 pagesThe Evolutionary Psychology of Men's Coercive SexualityJoão Marcos OliveiraNo ratings yet
- Full Download Gender Ideas Interactions Institutions 2nd Edition Wade Test BankDocument35 pagesFull Download Gender Ideas Interactions Institutions 2nd Edition Wade Test Bankepilepsyunabled.42qvq100% (34)
- Sexual Dimorphism Spatial Learning Metabolism Stress Diet (10-14)Document5 pagesSexual Dimorphism Spatial Learning Metabolism Stress Diet (10-14)Camila Alejandra Inostroza RiveraNo ratings yet
- Pandora BoxDocument11 pagesPandora BoxScribdTranslationsNo ratings yet
- Motivation and Emotion Summary NotesDocument147 pagesMotivation and Emotion Summary NotesluluuNo ratings yet
- 7.sex Differencies in Lateralisation in Animal BrainDocument224 pages7.sex Differencies in Lateralisation in Animal BrainIuliana OlteanuNo ratings yet
- Human Collective Aggression: A Behavioral Ecology PerspectiveDocument16 pagesHuman Collective Aggression: A Behavioral Ecology PerspectiveHector RafaelNo ratings yet