Professional Documents
Culture Documents
Lecture Notes MRI (Part2)
Uploaded by
cavronOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Lecture Notes MRI (Part2)
Uploaded by
cavronCopyright:
Available Formats
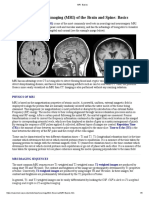
Medical Applications of MRI 2011 handout 1
1
Medical Applications of MRI
Dr John Thornton
e-mail: john.thornton@ucl.ac.uk
6 Lectures:
Review of nuclear magnetisation & the magnetic resonance signal
Conventional MRI:
Spin echo and gradient echo imaging
Proton density, T1, T2 and T2* image contrast
Fast Imaging Methods
Magnetization Transfer Contrast
Diffusion-weighted MRI
MRI Contrast Agents
MR angiography
MR Perfusion Imaging
Functional MRI
MRI Safety
Suggested Textbooks:
MRI From Picture to Proton, 2
nd
Edition (McRobbie, Moore, Graves and Prince, 2007)
Magnetic Resonance Imaging (Kuperman, 2000)
MRI Basic Principles and Applications, 4
th
Edition, (Brown & Semelka, 2010)
Terminology:
In these lectures:
Spins = nuclear dipoles = nuclei with magnetic moment = nuclear magnets
MR = (nuclear) magnetic resonance = NMR
Contrast = difference in image intensity for different tissues
= difference in numerical values for relevant pixels
difference in MR signal intensity originating from the each tissue
Overall Learning Objectives
By the end of these 6 lectures you should be able to understand and describe
the physical origin of image contrast in MRI
how scanner parameters may be selected to provide different types of medically useful contrast
How we can exploit the physics of magnetic resonance and tissue-water behaviour to provide
sophisticated structural and functional information
Medical Applications of MRI 2011 handout 1
2
Magnetization, RF pulses and the MR signal
Some nuclei (in particular the hydrogen nucleus, or proton) have the quantum mechanical property of spin and behave
as microscopic magnets.
In the presence of a magnetic field B
o
, on average, more nuclear spins align with the field than against and an
equilibriumtotal magnetisation (M
o
) parallel to B
o
is produced.
By convention the direction of B
o
(and therefore M
o
) defines the z axis
z
B
1
M
0
B
0
B
0
y
x
z
At equilibrium:
M
0
z
M
0
B
0
y
x
B
1
Apply a second magnetic field, B
1
, perpendicular to the z
axis and rotating at the Larmor frequency: = .B
o
To simplify things, we take a coordinate system
(the rotating frame) which rotates at the same
rate as B
1
:
B
1
now appears stationary
Medical Applications of MRI 2011 handout 1
3
B
1
exerts a torque on M
o
causing it to rotate down towards the x-y plane:
A B
1
field applied for a time just sufficient to rotate M
o
through 90
0
is termed a 90
0
pulse and generates a transverse
magnetization M
xy
Immediately following a 90
0
pulse, the longitudinal component of M, i.e. M
z
, is zero.
Viewed in the laboratory frame, M
xy
now rotates about the z axis with an angular frequency = -B
0
and therefore
induces a signal in a receiver coil.
The process of creating longitudinal magnetization (M
xy
) e.g. by means of a 90
0
RF pulse, is known as excitation.
Relaxation
after the 90
o
RF pulse the magnetization returns towards equilibrium (relaxes):
M
0
B
1
y
x
z
After 90
o
pulse |M
xy
| = |M
o
|
M
z
= 0
After a short time (typically a
few ms in MRI), M
o
is rotated by
90
o
onto the x-y plane
B
1
is then removed
This application of B1 in this
way
is known as a 900 RF pulse
NB The detected signal magnitude (and hence final image intensity) is proportional to |M
xy
|.
No |M
xy
|: no signal!
(M
z
is stationary both in the rotating frame and the laboratory frame, and therefore induces no
signal voltage in the receiver coil)
Immediately
following 90
o
pulse:
M
xy
= |M
o
|
Some time
later:
M
xy
< |M
o
|
M
z
Some more
time later,
t >> T
1
:
M
z
= M
o
Magnetization has returned to
equilibrium state
y
x
z
Medical Applications of MRI 2011 handout 1
4
The components of the magnetization before and after a 90
o
pulse may be represented graphically:
The longitudinal magnetization (M
z
) recovers to equilibrium exponentially with a time constant T
1
:
M
z
= M
o
.(1-exp(-t/T
1
)) (T
1
or spin-lattice relaxation) [eqn 1]
where t represents time from the end of the 90
0
pulse
The transverse magnetization (M
xy
) decays to zero with a time constant T
2
:
M
xy
= M
o
.exp(-t/T
2
) (T
2
or spin-spin relaxation) [eqn 2]
T
1
relaxation involves an exchange of energy between the spins and their environment (the lattice), causing the
relative populations in the up and down states to return towards their equilibrium distribution so that M
z
recovers
towards M
0
T
2
relaxation involves a gradual reduction in M
xy
by the above mechanism, and additionally because the spins interact
magnetically amongst each other (hence spin-spin) the net result of which is that they gradually lose phase coherence
(i.e. their individual magnetizations no longer all point in the same direction in the x-y plane) and hence their vector sum
(M
xy
) decays in magnitude with time
Because of this extra contribution to the decay of M
xy
, in biological tissue, T
2
< T
1
Inversion
Starting again from the equilibrium situation (|M
z
| = |M
o
|), the rotating B
1
field is applied for twice as long as for a 90
0
RF pulse.
Mtherefore continues to rotate past the x-y plane until it is oriented along the z direction.
This is known as an applying an 180
o
inversion RF pulse: immediately following this the magnetization is said to be
inverted.
The longitudinal magnetization then recovers exponentially towards equilibrium with time constant T1:
SOLUTIONS OF THE
BLOCH EQUATIONS
dM/dt = Mx B
after modification to
include relaxation
effects
Exponential recovery: time constant T
1
90
o
pulse applied here
M
o
M
xy
M
z
Exponential decay: time constant T
2
time
time
signal M
xy
Medical Applications of MRI 2011 handout 1
5
Following inversion, M
z
is described by the equation:
M
z
= M
o
(1-2exp(-t/T
1
)) inversion recovery (IR) equation
Here t represents time after the inversion pulse
Note: M
xy
is zero at all times in this case
Field inhomogeneities and the spin echo
In reality the main magnetic field, B
0
, is never perfectly uniform its value varies slightly with position. This B
0
inhomogeneity accelerates the decay of M
xy
: spins in different positions experience slightly different magnetic fields,
and thus precess at slightly different frequencies.
This frequency variation causes them to get out of step with each other , i.e. lose phase coherence, more rapidly than
they otherwise would such that their vector sum decreases more rapidly than would be caused by spin-spin (T
2
)
relaxation alone.
The signal decay is therefore described by a new time constant: T
2
* (< T
2
)
Such dephasing and consequent accelerated signal loss is usually a problem, but can be useful as it may reflect local
physiological changes - e.g. as in fMRI (see later).
Spin Echo Formation
This accelerated (T
2
*) decay due to field inhomogeneities is reversible by the applications of an 180
o
refocusing RF
pulse to form a spin echo:
Immediately
after inversion
pulse:
M
0
M
z
-M
0
M
0
Before
inversion
pulse:
Exponential recovery: time constant T
1
more time
later:
Some time
later:
time
y
x
z
Medical Applications of MRI 2011 handout 1
6
Signal at spin-echo peak
xy
(TE)= M
o
exp(-TE/T
2
)
Refocusing vs. inversion
Both refocusing and inversion involve an 180
0
RF pulse, but the effect is different because the magnetization is
orientated differently at the start of each pulse:
Inversion:
Start with magnetization along the positive z direction, M
z
= M
o
; M
xy
= 0. Apply B
1
pulse along +y in this case (180
0
y
)
M
0
B
1
y
x
z
180
o
refocusing pulse
(pancake flipper)
flips spins about x-axis
M
xy
Time constant
T
2
*
180
o
rf
pulse
Time constant T
2
180
o
M
xy
M
xy
Individual nuclei precess
at slightly different
frequencies
immediately after a 90
0
pulse
x
y
time t=0 t=TE/2 t=TE
Signal maximum occurs at the Echo Time (TE)
Signal,
S M
xy
Medical Applications of MRI 2011 handout 1
7
Refocusing:
Starts with magnetization in the x-y plane, i.e. M
z
=0; Individual spins have become out of phase: magnetization is
fanned-out in the x-y plane. Apply 180
0
B
1
pulse in this case along -x (180
0
x
), spins then tend to rephase.
Gradient echo formation
An echo signal may also be obtained without an 180
o
refocusing RF pulse by performing a field gradient reversal.
We deliberately introduce strong magnetic field inhomogeneities by applying a (negative) linear gradient. This greatly
increases the effective rate of T2* signal decay.
If the field gradient is then reversed, after an appropriate time the phase changes caused by the negative gradient are
exactly cancelled by the (opposite) phase changes caused by the positive gradient: a gradient echo is formed.
Note only phase differences due to the applied gradients are reversed: signal loss due to field inhomogeneities intrinsic
to the tissue (or scanner B
o
field) is not recovered and the amplitude of the gradient echo is determined by the natural
T2* decay rate.
Signal at gradient-echo peak M
xy
(TE)= M
o
exp(-TE/T
2
*)
TE
time
time
Gradient,
G
negative G
x
positive G
x
M
xy
Time constant
T
2
*
Area A
Area B
gradient-echo
occurs when
Area A = Area B
x
B
x
B
y
x
z
Dephasing occurs much more
rapidly than for the usual T2*
decay due to large additional field
inhomogeneities caused by the
applied gradient
Just after 180
o
pulse Just before 180
o
pulse
M
xy
B
1
y
x
z
M
xy
B
1
y
x
z
Rotate by 180
0
about
x axis
Medical Applications of MRI 2011 handout 1
8
The Spin-Echo Imaging Sequence
Both TE and TR are under software control and may be chosen by the operator to change the image contrast.
In general,
the magnitude and timing of the RF (B
1
) pulses determines the image contrast.
The field gradients G
x
, G
y
and G
z
provide spatial discrimination
These two aspects of image formation (contrast and localization) can be to a certain extent considered independently.
Image
contrast
Spatial
localisation
Medical Applications of MRI 2011 handout 1
9
MRI in medicine
Most clinical MRI is performed to provide structural & anatomical information: e.g. to detect atrophy, tumours, the
presence of haemorrhage, evidence of ischaemia (stroke) etc.
MRI examinations may also provide functional information relating to e.g. blood flow in vessels, cerebral activation
(fMRI), organ perfusion, uptake of tracer agents etc. giving additional information regarding tissue status
(viable/compromised/activated etc.)
Since the hydrogen nucleus (proton) has both the highest NMR sensitivity, and a very high concentration in the human
body (primarily as H
2
O), clinical MRI is concerned almost exclusively with imaging this nucleus, and therefore MRI scans
usually represent maps of the distribution of water in the body (there is also some contribution from protons in lipid
(fat) molecules ).
A primary source of contrast in MRI therefore relates to the concentration of hydrogen nuclei (proton density) in
particular tissue types. (NB the equilibrium magnetisation M
o
is proportional to the proton density).
As a consequence of differences in their microscopic environment protons in different tissue types exhibit different T
1
and T
2
relaxation times, e.g. in head imaging at 1.5Tesla approximate values are:
Protons associated with solid structures (e.g. compact bone) have T
2
relaxation times too short for them to be
detected in standard MR images and hence appear dark.
Because different tissues exhibit different relaxation times, and since pathology (tissue injury) can change these
relaxation times, MRI is said to have excellent soft tissue contrast.
By choosing suitable scan parameters, the image contrast may be manipulated to reflect these proton density and
relaxation time differences.
Contrast in conventional MRI
The signal (i.e. numerical value in each pixel) in a spin-echo image is given by:
Signal, S M
xy
= M
o
[1-exp(-TR/T
1
)].exp(-TE/T
2
)
By manipulating TR and TE we can change the signal dependency (weighting) with respect to M
o,
T
1,
and T
2
. This
generates different contrasts for tissues with different relaxation times and proton densities. We will now consider how
to generate images whose contrast is weighted in terms of the proton density, T
2
, T
2
* and T
1
of the tissues being
imaged.
TISSUE T
1
(ms) T
2
(ms)
white matter 600-800 60
grey matter 800-1000 80
fat 150 50
cerebrospinal 2700 500
fluid
Medical Applications of MRI 2011 handout 2
10
Signal Intensity in spin-echo imaging
The signal intensity in each pixel for a spin-echo image is given by:
S M
xy
= M
o
[1-exp(-TR/T
1
)].exp(-TE/T
2
)
S, the signal = numerical value of image pixel
or how dark or light the tissue appears in image
M
o
= the equilibriummagnetization
number of nuclei per unit volume
the concentration of water or fat molecules i.e. the proton density (PD)
Spin-echo imaging I: Proton density-weighted contrast
The proton density (PD) is proportional to the number of hydrogen nuclei (and hence water and lipid
molecules) in a given volume of tissue. M
o
is proportional to the PD. A proton-density-weighted image
gives contrast (relative signal intensity) dependent almost exclusively upon M
o
with very small or negligible
dependence upon T
1
or T
2
.
S PD.[1-exp(-TR/T
1
)].exp(-TE/T
2
)
T
1
= longitudinal (spin-lattice) relaxation time constant
T
2
= transverse (spin-spin) relaxation time constant
PD = the proton density
TE = the echo time
TR = the repetition time
Properties of the tissue
Scanner parameters
under control of the
operator
We can choose TE and TR to vary the amount by which S
depends upon T1, T2 or PD
Medical Applications of MRI 2011 handout 2
11
For PD weighting:
Signal M
xy
= M
o
[1-exp(-TR/T
1
)].exp(-TE/T
2
)
= M
o
Requires long scan times if TR is sufficiently long to completely remove T1 dependence (for which TR should
be ~ 5T
1
so that the spins are fully relaxed between 90
o
pulses) modern MRI systems use fast-spin
echo techniques to reduce scan time (see later).
N.B. PD weighted contrast may also be obtained using a gradient-echo pulse sequence with low flip angle
also see later.
Spin-echo imaging II: T2weighted contrast
We use a spin-echo sequence, this time with both long TR and long TE:
90
o
Spin-echo signal
Etc.
NB Abbreviated version of pulse-
sequence on page 8 not showing
the imaging gradients
Typical values:
TE ~ 10 ms
TR ~ 4000 ms
TR
TE
180
o
TE
TR long TE short
~ 0
~ 1
TE
TR
90
o
180
o
Spin-echo signal
Etc.
NB Abbreviated version of pulse-
sequence on page 8
T
90
o
180
o
Typical values:
TE ~ 100 ms
TR ~ 4000 ms
N.B sequence identical to previous figure,
except TE longer!
TR
Etc.
Medical Applications of MRI 2011 handout 2
12
For T2 weighting:
Signal M
xy
= M
o
[1-exp(-TR/T
1
)].exp(-TE/T
2
)
Therefore to a good approximation:
Signal M
xy
(TE) = M
o
.exp(-TE/T
2
)
and 2 tissue types (e.g. white and grey matter in the brain) having different T
2
values will give different
image signal intensities even if they have identical PDs.
Increasing the echo time (TE) increases the degree of T
2
signal dependence (i.e. increases the "T
2
weighting"
or T
2
contrast).
Note the image signal intensity in the above equation is still dependent upon M
0
(i.e. PD), but the image is
additionally T
2
weighted. Since the PD (and hence M
0
) may not vary much between different soft tissues,
with sufficiently long TE theT
2
effect predominates.
Like PD-weighted spin-echo imaging, T
2
-weighted imaging requires long scan times if we wait for complete
relaxation to completely eliminate T
1
influences (i.e. TR ~ 5T
1
as above), but scan times are again
commonly decreased by using the fast spin-echo method (see later).
Spin-echo imaging III: T1weighted contrast by progressive saturation
We use a spin-echo sequence this time with a short TR (<T
1
) such that relaxation recovery of M
z
between
excitations (90
o
RF pulses) is incomplete.
We focus on the behavior of the longitudinal magnetization M
z
which does not have time to fully relax back
to M
0
in between 90
o
pulses.
TR long TE long
~ 0
< 1
Echo time (TE)
Signal
Signal from tissue 2
(short T2)
Signal from tissue 1
(long T2)
TE short: signal difference (i.e. T2
contrast) small
TE long: signal difference (i.e. T2
contrast) large
This plot shows the situation
for 2 tissues with identical
proton densities
Medical Applications of MRI 2011 handout 2
13
In the steady state (once the sequence has been running long enough for an equilibrium situation to have
been developed) the signal intensity is again given by the spin-echo signal equation. This time the echo time
TE is made as short as possible, so the signal has very little dependence upon T
2
:
Signal, S M
xy
= M
o
[1-exp(-TR/T
1
)].exp(-TE/T
2
)
~ M
o
[1-exp(-TR/T
1
)]
So image contrast now depends on M
0
and T
1
, and for sufficiently short TR, the T
1
dependence (contrast)
dominates. In this case signal the intensity of longer T
1
tissues is preferentially decreased.
Advantage of this approach: short TR means T
1
-weighted images can be produced in a relatively short
acquisition time.
Spin-echo imaging IV: inversion recovery imaging
We can add an extra 180o inversion pulse to the start of each repetition of a spin-echo imaging sequence.
We then wait for a time TI (the inversion time) after this pulse before continuing with the rest of the spin-
echo imaging sequence. This introduces a different kind of T1 signal dependence.
Each 90
0
pulse shown is in fact
the first RF pulse for each
repetition of the spin-echo
imaging sequence
As usual the phase encode
gradient in incremented for
each repetition
Repeat until the required number of phase
encoding steps have been acquired
Short T1
tissue
Long T1
tissue
time
Also known as a progressive saturation pulse sequence
90
o
90
o
90
o
90
o
TR
90
o
90
o
M
z
~ 1 (v. short TE << T
2
)
Medical Applications of MRI 2011 handout 2
14
During the inversion time TI there is partial recovery of M
z
towards M
0
by T
1
relaxation. Again we consider
behaviour of M
z
:
Repeat e.g. 256 times with increase phase encoding gradient
TI
slice
select
Phase
encode
read
90
o
180
o
Spin echo
180
0
inversion
pulse
Repeat until the required
number of phase encoding steps
have been acquired
180
0
T
1
contrast
90
o
M
0
M
z
-M
0
TR
TI
long T1 tissue
shortT1 tissue
time
T
1
contrast
90
o
Again follow each 90
0
pulse
with an 180
0
refocusing pulse
and apply imaging gradients
Phase encode gradient
incremented for each repetition
Medical Applications of MRI 2011 handout 2
15
The full expression for the signal intensity is:
S M
xy
= M
o
[1-2.exp(-TI/T
1
)+ exp(-TR/T
1
)].exp(-TE/T2)
For long TR (>> T
1
) and very short TE (<< T
2
), this simplifies to
S(TI) M
xy
= M
o
(1-2.exp(-TI/T
1
))
S depends heavily on T
1
- inversion recovery imaging can give strong T
1
contrast
Selective Nulling
Note that the IR method forces the longitudinal magnetization to pass through zero. If the image data is
acquired at the exact time when M
z
for a particular tissue passes through zero we get no signal for this
tissue it has been nulled and will appear black in the final image.
This occurs at TI = ln(2).T
1
= 0.693T
1
We can selectively eliminate signals from specific tissues in this way:
e.g. STIR (Short TI inversion recovery): reduces the signal from fat tissue (fat suppression)
Fat has a T
1
of approx. 200 ms at 1.5T. Therefore the inversion recovery curve for fat tissue
passes through zero at 0.693x200 = 139 ms
FLAIR (Fluid Attenuated Inversion Recovery) suppresses long T
1
fluids e.g. cerebrospinal
fluid (CSF)
180
0
inversion
pulse
90
o
90
o
M
z
time
Short T1 tissue
long T1 tissue
Acquire image here:
null short T
1
component
Acquire image here:
null long T
1
component
Medical Applications of MRI 2011 handout 2
16
Advantages of inversion recovery sequence for T1-weighted imaging:
There a greater dynamic range of potential T
1
contrasts available due to relaxation fromM
o
to + M
o
(compared with progressive saturation)
Selective nulling is possible (see above)
Disadvantage: long scan times due to long TR requirement (usually mitigated using fast-spin echo
methods).
(In addition to progressive saturation and inversion recovery techniques, T1-weighted imaging may also be
obtained using a gradient-echo imaging sequence - see later).
CONTRAST AND NOISE
An objective in medical is to maximize contrast between different tissues, e.g. between healthy and
diseased tissue, while minimizing the deleterious effects of image noise.
To separate features in our object we must have image contrast, here defined as a difference in image
signal intensity between tissue types or between normal/abnormal tissue in the same organ.
As already mentioned, the image signal in MRI is always dependent to a certain extent upon M
o
(i.e.
proportional to the proton density), but usually the image siganl is also made to depend upon (weighted in
terms of) other factors e.g. T
1
or T
2
(and other types of contrast to be described later).
Our aim is always to maximise signal contrast, but we must also take into account the influence of noise in
the imaging system.
Consider the case of a T
2
-weighted sequence for 2 tissues with identical M
0
but different T
2
s. (Similar
considerations apply for other contrasts e.g. T
1
-weighted contrast etc.). The graph shows how the tissue
signal levels depend on the choice of echo-time, TE.
There is a TE which gives a maximum difference between the curves and therefore images collected with
this TE will show the greatest contrast.
Maximum
Signal difference (contrast)
Time (TE)
Signal
Signal from tissue 2:
short T2
Signal from tissue 1:
long T2
Noise level 1
Noise level 2
Medical Applications of MRI 2011 handout 2
17
However, we have to consider the effects of image noise. Noise in MRI originates largely from the random
thermal motion of ions within tissue, which induce random electrical signals in the MRI receiver coil. The
precise mean noise level in an image depends upon a number of factors, including the volume of tissue
within the receiver coil and the receiver bandwidth.
At the echo-time giving the maximum signal difference the signal-to-noise ratio may be poor: e.g. if the
noise were at Noise Level 2 in the example in the diagram, the image would be largely obscured by noise
and diagnostically useless. Thus in order to obtain the most diagnostically sensitive images, a compromise
between contrast and SNR may be required, and we aim to maximise the contrast-to-noise ratio (CNR)
defined as
CNR = (S
1
-S
2
)/S
noise
where S
1
and S
2
are the signals from tissues 1 and 2, and S
noise
is the mean noise level.
S
1
/S
noise
and S
2
/S
noise
are the signal to noise ratios (SNRs) for the 2 tissues.
Image noise affects imaging performance in 2 ways:
1. it limits the minimum signal levels which may be detected
2. it may also limit the maximumimage contrast which may be obtained
For a particular pulse sequence, the objective is always to select pulse sequence parameters which
maximize the contrast-to-noise ratio for the tissues/pathologies in which we wish to discriminate.
Medical Applications of MRI 2011 handout 2
18
Fast MRI Methods
Typically, a standard spin-echo imaging sequences would take 5 - 10 minutes to acquire an image data set.
(image acquisition time = TR x number of phase encoding steps)
It is often desirable to collect images more rapidly (with imaging times potentially < 1 minute, possibly < 1
second):
- to reduce examination duration (reduce patient distress and increase patient throughput)
- to achieve high spatial resolution in 3 dimensions
- to freeze the effects of motion (e.g. cardiac MRI, abdominal imaging)
- to collect images with a high temporal resolution for dynamic imaging (e.g. contrast agent studies,
fMRI)
We will consider 3 rapid MRI techniques: gradient-echo imaging, fast spin-echo imaging and echo-planar
imaging. With contemporary equipment these methods are routinely available and often used in both
clinical and research scanning.
Fast Imaging I: Gradient Echo Imaging
Variants are known as FLASH (Fast Low Angle Shot) or SPGR (SPoilt GRadient echo) or FFE (Fast Field Echo)
By way of introduction, let us compare compare the z magnetization behaviours in PD-weighted
versus T
1
-weighted spin-echo imaging sequences:
PD-weighted:
The image signal is proportional to M
xy
immediately after each 90
o
RF pulse, |M
xy
|
after 90
o
= |M
z
|
before 90
o
For a PD-weighted sequence (long TR), , M
xy
|after 90
o
~ M
0
, i.e. signal is maximum available
However, for 256 phase-encoding steps, total imaging time is 256 x 4s ~ 17 minutes
M0
Mz
90
o
90
o
time
90
o
Long TR (e.g. 4s)
Medical Applications of MRI 2011 handout 2
19
T1-weighted:
We use a shorter TR to increase the T1 weighting in the final image
TR is too short to allow complete T
1
relaxation, so
|M
xy
| after 90
o
= |M
z
| before 90
o
<< |M
0
| and the image signal is much reduced
However, scanning time is now faster: 256 x 0.5s ~ 2 minutes
To scan even faster:
We might try drastically reducing TR to decrease the scan time even further.
However, this would cause the remaining signal to be too small too be useful (remember the importance of
maintaining an adequate contrast-to-noise ratio):
In this case, |M
xy
| after 90
o
(= |M
z
| before 90
o
) is so small that signal would be below the noise level,
and therefore useful images may not be obtained in this way.
M
z
immediately prior to each RF pulse, and hence the subsequent signal, may be increased by reducing the
disturbance from equilibrium at each repetition, i.e. by using an excitation pulse with a flip angle < 90
o
:
M
0
M
z
90
o
90
o
90
o
90
o
time
Very short TR (e.g. < 0.1s)
short TR (e.g. 0.5s)
M
0
M
z
90
o
90
o
90
o
90
o
time
Smaller M
z
< M
o
therefore smaller
signal
Medical Applications of MRI 2011 handout 2
20
e.g. immediately following a 30
o
RF pulse
M
z
= M
Zstart
.cos(30
o
) = 86.6 % of |M
Zstart
|
M
xy
= M
Zstart
.sin(30
o
) = 50 % of |M
Zstart
|
i.e. we can generate significant M
xy
without reducing
M
z
by very much.
Considering again for comparison the case of 90
0
RF pulses applied with short TR:
This is effectively equivalent to a very heavily T
1
-weighted progressive saturation sequence (as discussed
earlier), where, assuming a TE sufficiently short to ignore T
2
effects has been chosen,
Signal M
o
[1-exp(-TR/T
1
)]
For low flip-angle RF pulses, i.e. flip angle o < 90
o
, solution of the Bloch equations shows that the
equilibrium value of M
z
immediately prior to each RF pulse is greater than for the case of 90
o
pulses, and
hence the available signal is also greater.
M
0
M
z
o
o
o
o
o
o
o
o
time
Very short TR (e.g. < 0.1s)
M
Zstart
B
1
y
x
z
M
z
M
xy
o = 30
o
M
0
M
z
90
o
90
o
90
o
90
o
time
Very short TR (e.g. < 0.1s)
Medical Applications of MRI 2011 handout 2
21
The signal is now given by a more general equation:
S M
o
[1-exp(-TR/T
1
)].sin(o) (again assuming v. short TE)
1-cos(o) exp(-TR/T
1
)
A maximum signal for a tissue with given T
1
is obtained at a specified TR by using a flip angle
satisfies the equation:
cos(o) = exp(TR/T
1
) and o is in this case known as the Ernst angle.
In order to further decrease the total acquisition time, the signal is acquired using a gradient echo
(saving the time required for a refocusing pulse and its associated field gradients), so that a shorter
TR may be used in order to minimize the time taken to acquire the image:
Example: for a tissue with T
1
= 800ms; TR = 100ms
o = 90
o
: Signal M
o
[1-exp(-TR/T
1
)] = 0.12 x M
0
o = 30
o
: Signal M
o
[1-exp(-TR/T
1
)].sin(30
o
)
1-cos(30
o
) exp(-TR/T
1
)
= 0.25 x M
0
i.e. signal strength doubled !
Also assumes sequence is
spoilt see below
Medical Applications of MRI 2011 handout 2
22
Because the TR is short compared with T
2
, immediately before each o
0
RF pulse there may remain a
substantial transverse magnetization, M
xy
(in a spin-echo sequence, this would normally have
decayed to zero). Left alone, this magnetization might interfere with the transverse magnetization
produced by the subsequent RF pulses, causing errors in the image formation process. The purpose
of the crusher gradients is to avoid this by completely dephasing the transverse magnetization, such
that the vector sum of all the individual nuclear moments contributing to M
xy
is zero before the next
RF pulse is applied.
TE
o
0
, slice selective
RF pulse
gradient echo
acquire
o
0
slice select
gradient
read
gradient
Phase encoding
gradient
spoiler gradients
ensure M
xy
= 0 before
next o
0
pulse
TR (short !)
Phase encode
gradient
incremented for
each repetition
Repeat number
of phase encoding
steps
RF/signal
Medical Applications of MRI 2011 handout 2
23
Digression: T2*-weighted imaging
For simplicity, the above treatment assumed that the echo time TE was very short. More generally,
for a gradient-echo pulse sequence, explicitly including the TE:
S M
o
[1-exp(-TR/T
1
)].sin(o) . exp(-TE/T
2
* )
1-cos(o) exp(-TR/T
1
)
If TR is long, and o small, this simplifies to: S M
o
. exp(-TE/T
2
* )
For longer TEs (say 40ms) we can obtain contrast that depends upon T
2
* which is sensitive to local
disturbances of the magnetic field homogeneity, e.g. a local build up of iron in tissue as may occur in
blood and blood-breakdown products following bleeding (haemorrhage).
[Fast T
2
*-weighted images may also be obtained using echo-planar imaging see later]
Advantages of gradient echo imaging:
- Fast: Imaging times less than 1 minute possible
- can also obtain true 3D scans in reasonable examination times ( < 10 minutes)
- versatile: image contrast can be selected by choice of o, TR and TE (the sequence
may additionally be preceded by an 180
o
inversion RF pulse to provide additional T
1
-
weighted contrast)
Disadvantages of gradient echo imaging:
- SNR may be low (but can be improved by signal averaging)
- lack of 180
o
refocusing pulse may result in susceptibility artefacts.
Gradient echo imaging: summary
Signal , S M
o
[1-exp(-TR/T
1
)].sin(o) . exp(-TE/T
2
* )
1-cos(o) exp(-TR/T
1
)
Appropriate choice of o, TR and TE determines contrast:
Short TR (~ 100ms), Short TE, and/or large o (~ 80
0
)
T
1
weighting
Moderate TR (~ 500ms), short TE and small o (10-20
0
)
PD weighting
long TE (~ 40ms), moderate TR (~ 500ms) and small o (10-20
0
)
T
2
* weighting
Medical Applications of MRI 2011 handout 3
24
Fast Imaging II: Echo Planar Imaging (EPI)
In both the gradient- and spin-echo imaging pulse sequences, a series of n (= number of phase
encoding steps) gradient- or spin-echoes are acquired one at a time. For each acquisition the phase-
encoding gradient is increased by a fixed amount. Total imaging acquisition time is n TR.
In EPI, the equivalent dataset is acquired in a single shot following a single RF excitation pulse.
A slice-selective 90
o
RF pulse is applied, followed by reversal of the read gradient to form a gradient
echo in the usual way. After the acquisition (digitization) of this gradient echo, the read gradient is
reversed again causing a second gradient echo to form. This process is repeated to form a train of,
say, 64 gradient echoes which are all separately digitized.
Before the first of these gradient echoes is acquired, a large negative phase-encoding gradient is
applied. A small positive phase-encoding gradient (blip) is switched on in between subsequent
gradient echoes.
At the time of each gradient echo, the nuclear spins have experienced the cumulative sum (i.e. time
integral) of the preceding phase encoding gradient pulses. Therefore the first gradient echo has
experienced a large negative phase-encoding gradient, the second effectively a smaller negative
phase-encoding gradient (the initial large negative excursion + the first positive blip), the third a
smaller again negative phase-encoding gradient (the initial large negative excursion + the first
positive blip + the second positive blip) and so on.
By the time of the final gradient-echo acquisition, the effective cumulative phase-encoding gradient
has stepped from the initial large negative value to a large positive value.
From the point of view of phase-encoding, the data set is equivalent to that which would be
obtained if each gradient-echo were acquired in completely separate acquisitions, with the phase
encoding stepped between each one, as happens in a standard spin- or gradient-echo imaging
sequence.
Thus complete 2-dimensional image information is built up in a single shot following a single 90
o
RF excitation pulse. This may be achieved in times of the order of 100 ms.
Medical Applications of MRI 2011 handout 3
25
An echo-planar imaging pulse sequence
The EPI sequence as shown is based entirely on gradient echoes, and therefore produces images
with T
2
*-weighted contrast. Alternatively, the addition of a single 180
0
RF refocusing pulse before
the read gradient is switched on for the first time gives an image with T
2
-weighted contrast.
The EPI sequence may be preceded by a contrast preparation module (e.g. an 180
0
inversion pulse
to generate T1-weighting, or diffusion-weighting gradients (- see later)) to generate specific tissue
contrasts.
90
0
R.F
Slice
gradient
read
gradient
Phase-
encoding
gradient
signal
Gradient
echoes
Equal
areas
Equal
areas Keep repeating read
gradient reversal until
all phase encode steps
acquired, e.g. 64 times
Equivalent to in other sequences
Medical Applications of MRI 2011 handout 3
26
Advantages of EPI:
- can "freeze" subject motion
- high temporal resolution : useful for fMRI or perfusion imaging (see later)
- high signal-to-noise ratio per unit time
Disadvantages of EPI:
- rapid gradient switching requires high-performance system hardware
(however, modern all MRI systems are generally EPI-capable)
- rapid gradient switching generates large amount of acoustic noise
(although not necessarily worse than other high-speed MRI methods)
- gradient switching rate limits resolution (typically 64 x 64 pixels or 96x96)
- magnetic susceptibility variations can cause image distortion and signal loss
Fast Imaging III: Fast Spin-Echo Imaging (FSE)
(Also known as Turbo Spin-Echo imaging (TSE)). The principle here is similar to EPI: a series
of separately phase-encoded echoes is generated following a single 90
0
excitation pulse, but this
time a train of 180
0
refocusing pulses are added to form a series of spin echoes rather than the
gradient echoes used in EPI (see diagramon next page).
Medical Applications of MRI 2011 handout 3
27
FSE sequence: schematic diagram for 3 180
0
refocusing pulses
90
o
180
o
refocusing
RF pulse
90
o
RF
pulse
Signal M
xy
M
x
y
M
x
y
180
o
M
x
y
180
o
M
x
y
180
o
180
o
refocusing
RF pulse
Spin Echo
180
o
refocusing
RF pulse
Spin Echo
Spin Echo
Behaviour of M
xy
Phase encoding gradient
Equal areas, opposite polarity
[positive pulses cancel out phase
changes caused by original negative
phase encoding gradient, so that the
phase encoding is reset to zero
before each 180
0
pulse].
Phase encoding gradient incrementing for each
acquired echo as in previous sequences
Equal areas, opposite polarity
Medical Applications of MRI 2011 handout 3
28
In a FSE sequence, a number (typically 8) of 180
0
RF refocusing pulses are applied after each
90
0
pulse, producing 8 spin-echoes per repetition period. Each spin echo is generated and digitized in
the presence of slice select and read gradients as described previously. Crucially each spin echo also
experiences a phase-encoding gradient pulse, the magnitude of this gradient being unique for each
echo. In this way, 8 phase encoding steps are collected for each repetition. After a recovery delay TR,
a second set of 8 spin-echoes is acquired in exactly the same way except that in this case the value of
each of the phase-encoding gradients are different from those in the first set. This process is
repeated until all the phase encoding steps (typically 256) have been acquired. In the case of a
256x256 pixel image acquisition, for a standard spin-echo imaging sequence, the total imaging time is
256 x TR. For a FSE sequence however, the total imaging time will be (256/8) x TR = 32 x TR, i.e. the
imaging time is reduced by a factor of 8. For each of the 32 individual acquisitions, different phase
encoding gradient magnitudes are used, so that, at the end 256 spin echoes have been acquired,
each having experienced a unique phase encoding gradient.
Important difference between EPI and FSE imaging: In FSE imaging, each echo is followed by a phase-
encoding gradient pulse equal in area but of opposite polarity to that preceding it. This effectively
resets the phase encoding after each spin echo, so that the phase encoding gradient affecting a
particular echo is independent of the phase encoding for previous echoes; in EPI the phase encoding
affecting each echo is the cumulative sum of the preceding phase encoding gradient pulses.
T
2
-weighted FSE, sometimes with an additional initial 180
0
inversion RF pulse for FLAIR suppression
of the CSF signal, are commonly used for clinical investigations in the brain.
Advantages of FSE imaging
High resolution (c.f. EPI) 256x256 or 512x512 pixels per image possible
Good T2 or PD contrast depending upon order of phase-encoding steps
Good resistance to magnetic susceptibility problems
Disadvantages of FSE imaging
Not as fast as EPI FSE is a multi-shot sequence
Lots of 180
o
RF pulses
Medical Applications of MRI 2011 handout 3
29
Summary: conventional MRI images with PD, T
1
,T
2
or T
2
* based contrast
T
1
Contrast PD Contrast
Progressive saturation Standard spin-echo sequence
(short TR/TE spin echo sequence) (long TR, short TE)
Inversion recovery Gradient echo sequence
(Inversion pulse preceding short (typically short TR, short TE, low )
TE/long TR spin-echo sequence)
Gradient echo sequence FSE sequence
(with short TR, short TE, high ) (long TR, short effective TE)
T
2
Contrast T
2
* Contrast
Standard spin-echo sequence Gradient echo sequence
(long TR, long TE) (typically moderate TR and , long TE)
FSE sequence EPI without 180
o
refocusing pulse
(long TR, long effective TE) (long TE gradient echo)
EPI with 180
o
refocusing pulse (long TE)
Beyond Conventional MRI Other Contrast Mechanisms
T
1
, T
2
, T
2
* and PD provide excellent soft-tissue contrast, and these quantities change with many
pathologies, and so provide image contrast which is diagnostically useful. The use of sequences to
provide this type of contrast has been referred to as conventional MRI
However, changes in these quantities may be non-specific, and tend reflect long-term tissue injury
rather than recently occurring (acute) changes.
Methods have therefore been developed which exploit other contrast mechanisms that more
directly reflect the physiological status of tissue, we will now explore some examples of theses.
1. Magnetization Transfer Contrast (MTC)
The spin-spin (T
2
) relaxation of water molecules is influenced by random magnetic field
fluctuations, largely due to the tumbling motion of neighbouring (magnetic) molecules. Populations
of molecules which are free to tumble have long T
2
relaxation times, whereas water molecules which
are bound to e.g. membranes or macromolecules are less free to tumble and hence have short T
2
relaxation times.
In tissue (e.g. brain or muscle), water molecules may be considered to exist in one of two
microscopic environments: either bound to macromolecules or cell membranes such that their
rotational motion is restricted, or free to undergo rotational motion, e.g. water in the cytosol. The
T
2
of bound molecules is very short (< 1 ms) so that they are invisible to conventional MRI.
MTC imaging is a way of observing the effects of these bound molecules indirectly.
Medical Applications of MRI 2011 handout 3
30
Fourier theory predicts an inverse relationship between the relaxation time (decay) constant
(i.e. T
2
) in the time domain, and resonance line width in the frequency domain:
A series of RF pulses applied "off-resonance" (i.e. B
1
rotating with angular frequency different from
that of the rotating proton transverse magnetizations M
xy
) have no direct effect on the free water,
because their frequency lies outside the resonant frequency range for these spins. However, because
the RF frequency lies within the line width of the bound water, the magnetization of this water is
reduced to close to zero (saturation). Under certain circumstances, e.g. due to physical exchange,
chemical exchange or magnetic interactions (dipolar coupling) between spins in the bound-water and
free-water populations, (i.e. magnetization transfer) the magnetization of the free water may also
become reduced, and hence the MRI signal intensity is decreased:
Therefore if off-resonance (1-5 kHz) saturation pulses are added to conventional imaging sequences,
tissues which have a significant fraction of bound water molecules will show a reduced image
intensity. Thus MTC provides additional contrast between tissues containing complex microstructure
(larger bound water fraction, e.g. brain tissue), and those which dont (e.g. blood).
r
e
s
p
o
n
s
e
frequency
Fourier
Transformation
bound water molecules
- short T
2
time
S
i
g
n
a
l
free water
molecules
- long T
2
free water
molecules
- narrow resonance
bound water molecules
- broad resonance
CASE 1: No transfer of magnetization
between free and bound water
The off-resonance
pulse has no effect on
the free water pool
Apply off-resonance
saturation pulses
The bound pool
magnetization is reduced
RF pulses
CASE 2: No transfer of magnetization
between free and bound water
Free water magnetization
reduced due to exchange
of magnetization between
free and bound pools
RF pulses
Medical Applications of MRI 2011 handout 3
31
Applications of MTC:
1. Selective suppression of "solid" tissue, e.g. for magnetic resonance angiography where signal
from brain tissue is suppressed in order to highlight that from free water in the blood (see
later).
2. Investigation of conditions in which tissue micro-structure is disturbed, e.g. in demyelinating
diseases such as multiple sclerosis, the solid/free water dynamics are altered such that areas
of diseased brain tissue show changes in MTR.
In this type of application it is common to produce maps of the magnetization transfer ratio
(MTR): A control image is acquired without off-resonance saturation pulses, and a second
image is acquired with the off-resonance saturation pulses, and for each pixel the ratio of the
value from each of the acquired images is calculated.
Medical Applications of MRI 2011 handout 4
32
Beyond Conventional MRI Contrast Mechanisms
2: Diffusion Weighted Imaging (DWI)
Water diffusion in tissue
Water molecules undergo random thermal motion or self diffusion, familiar as "Brownian Motion".
After a time t an individual water molecule following a random walk will have achieved a total
displacement from its start position which may be represented by a vector r.
Considering a large ensemble of water molecules, after a certain time t, adding all the displacements
together the mean vector displacement is zero molecules are equally as likely to have experienced
a positive as a negative displacement.
However, the mean squared displacement (for 3 dimensions) is given by:
<r
2
> = 6Dt Einstein relation
where t is the observation time and D is the diffusion coefficient.
For free water, D = 2.0 x 10
-3
mm
2
.s
-1
, therefore if t = 100 ms, the root mean square displacement
(\(<r
2
>)) ~ 35 m. In tissue, cellular structures have dimensions < 100m, so that over this
timescale, the free motion of water molecules is restricted. This causes the measured diffusion
coefficient to be reduced compared with free water. Therefore in tissue instead of D we refer to the
Apparent Diffusion Coefficient (ADC < D). The degree to which diffusion is reduced in tissue is a
reflection of tissue microstructure, and hence if MRI can be made sensitive to diffusion effects,
diffusion may provide a useful source of contrast, changing with tissue type and pathology.
Generating diffusion-weighted contrast in MRI
A standard spin-echo sequence is modified with the addition of diffusion sensitising gradient pulses:
(cont. on next page)
r
r
Position at time 0
Position at time t
After a time t individual molecules in a particular
volume have undergone displacements (r) with
random directions and lengths
Therefore summing over all molecules in the
volume, the mean displacement, <r> = 0
Medical Applications of MRI 2011 handout 4
33
|
final
|
2
|
1
|
1
|
1
x x
x
y y y y
x
Period 1
Consider a water molecule at position x
1
during
period 1. During this interval,
e
1
= .G
diff
.x
1
At the end of period 1, the phase acquired by a
spin at position x
1
during Period 1 is,
|
1
= e
1
.t
Period 1
= .G
diff
.x
1
.o
time
G
diff
Period 1
G
diff
180
0 90
0
Spin echo
Period 2
o
o
A
x
1
x
B
x
2 x
B
Period 2
Imagine that the water molecule now moves so
that it is at position x
2
during period 2. During
this interval,
e
2
= .G
diff
.x
2
the additional phase acquired by a spin at
position x
2
during Period 2 is,
|
2
= e
2
.t
Period 2
= .G
diff
.x
2
.o
The total phase for a particular spin at the peak of the spin echo must be calculated taking into account
the effect of the 180
0
refocusing pulse, which rotates the spins by 180
0
about the x axis:
After 90
0
pulse:
At the end
of period 1:
After 180
0
pulse:
At the end
of period 2:
|
final
= |
2
- |
1
= .G
diff
.o.( x
2
x
1
)
During periods 1 and 2, if the gradient G
diff
is applied in the x direction, in the rotating frame, B = G
diff
.x and
therefore the angular frequency of spins depends upon position: e= .G
diff
.x
The presence of the diffusion gradients affects the amplitude of the spin-echo obtained. To see how this is so,
consider the following highly-simplified model of water molecule motion. Remember:
i) Magnetization in the x-y plane, M
xy
, rotates about the z axis with angular frequency e = B
0
ii) By the phase of M
xy
at any instant, we mean the angle between M
xy
and (say) the x axis at that point in time
iii) This phase angle, | = et, where t is the time from the start, and
x
y
Medical Applications of MRI 2011 handout 4
34
For stationary spins, |
1
= |
2
, and as a result of the 180
o
pulse, the phase advance acquired during the
first diffusion gradient pulse (Period 1) is cancelled out by that produced by the second diffusion
gradient pulse during Period 2, and the total phase gained (|
final
) is zero (c.f. spin echo formation)
For moving spins, the phase gained during the second diffusion gradient pulse is not equal to that
acquired during the first, and a net phase difference, |
final
, remains at the echo time.
In reality, the diffusion motion of spins is more complex, but the same basic principle holds. Since the
diffusive motion of spins is random, the phase advance, |
final
, gained by individual spins is randomly
distributed (because e.g. the values of x
1
and x
2
in each case are randomly distributed), and hence at
the echo-time the individual magnetization vectors point in different random directions in the x-y
plane, and their vector sum, and hence the final spin-echo signal magnitude, is reduced:
The more rapidly the spins diffuse, the greater the range of the individual x
1
s and x
2
s, the larger the
phase dispersal between individual spins, and the smaller their final vector sum M
xy
.
Quantitatively, for general simple self-diffusion, the spin-echo signal magnitude is given by:
S = S
o
.exp(-b.D)
b =
2
G
diff
2
o
2
(Ao/3)
where S
o
is the signal magnitude obtained without applying the diffusion gradients, is the
magnetogyric ratio, G
diff
is the diffusion gradient strength and o and A are respectively the duration
and separation of the leading edges of the diffusion gradient pulses (see diagram at top of previous
page).
b is known as the "diffusion weighting" (units s.m
-2
) or b factor
(typically diffusion weighting = 1000x10
6
s.m
-2
, sometimes expressed as a b factor of 1000)
Increasing b (by increasing G
diff
, o or A) increases the diffusion-weighting of the image.
Incorporating the diffusion-weighting gradients into a spin-echo imaging sequence in this way
produces diffusion-weighted images in which water which is free to diffuse gives a low signal, and
water which is less mobile gives a high signal. Repeating the image sequence with increasing values
of b allows the actual ADC in each pixel to be estimated and the calculation of maps of ADC. For
example, a diffusion-weighted image is collected with diffusion weighting b = 1000x10
6
s.m
-2
(b
1000
)
and a second which identical except the diffusion weighting is zero (b
0
). If, for a given pixel, the
Resultant
magnetization < |M
0
|
Slow diffusion: Rapid diffusion:
Resultant
magnetization << |M
0
|
Medical Applications of MRI 2011 handout 4
35
image intensities for the two images are given by S
1000
and S
0
respectively, then the ADC for that pixel
is given by:
ADC = -1/(b
1000
-b
0
) . ln(S
1000
/S
0
)
= -1/1000x10
6
. ln(S
1000
/S
0
)
ADC maps are useful because they eliminate the T2 and PD weighting present in the source images,
and provide quantitative information directly comparable between patients.
Clinical Application of DWI: Cerebral ischaemic injury (stroke):
Cerebral ischaemia means loss of blood supply to the brain. This means the cells will receive
insufficient oxygen etc. and are unable to maintain osmotic homeostasis i.e. they swell up. This
causes a bigger fraction of the tissue water to be located inside the cells, where diffusion is lower,
and therefore the average ADC is reduced. ADC reduction occurs within minutes of ischaemia (c.f. T
2
changes which may take hours or days to occur following ischaemia). DWI is widely used in clinical
MRI to assess acute stroke and other ischaemic conditions.
Diffusion Anisotropy and Diffusion Tensor Imaging
The diffusion of free water is said to be anisotropic: there is no preferential direction for the
molecular motion: motion in all directions is equally probable.
However, the diffusion of water in tissue is impeded by cell membranes and other structures.
Therefore, taking as an example white matter nerve bundles in the brain, the ADC measured with
diffusion-gradient directions applied parallel to the nerve fibres main axis appears higher than that
measured with diffusion-gradient directions orthogonal to the fibre directions.
Therefore the measured ADC depends on the relative geometric orientation of the tissue and the
measurement gradient direction.
neuron
High
diffusion
(ADC
parallel
)
Low diffusion
(ADC
orthogonal
)
Medical Applications of MRI 2011 handout 4
36
If diffusion is anisotropic, it can no longer be adequately described by a simple scalar diffusion
coefficient (i.e. the ADC). In the most general case the diffusion behaviour is described by a tensor. A
tensor is a matrix of values. The diffusion tensor is 3 x 3 matrix, containing 9 values which
characterise the interactions between the diffusion gradient orientations and the 3-dimensional
directional dependence of the molecular diffusion. The diffusion tensor (DT) is written as:
The details are complicated and beyond the scope of these lectures, but if at least 6 diffusion-
weighted images are acquired, each with the diffusion-sensitizing gradients applied in a different
direction, together with one image with no diffusion weighting (b=0), then it is possible to calculate
all the elements of the DT for each image pixel, and calculate a vector representing the principle
direction of the underlying diffusion. The direction of this vector for each pixel in the image indicates
the direction of the white matter fibres in this volume of tissue. By comparing the direction of this
vector in neighbouring pixels it is possible, e.g. by calculating streamlines, to reconstruct the path of
underlying white matted tracts: this is the principle of white matter tractography.
seed voxel
Alternatively, to obtain a measure of diffusion which is independent of direction, a simple scalar
quantity analogous to the ADC discussed above, then we can calculate the mean diffusivity (MD),
given by:
MD = (D
xx
+ D
yy
+ D
zz
)/3
Diffusion Weighted Imaging Pulse Sequences
In practice DWI (and DTI) are performed by adding the diffusion-weighing gradients to a spin-echo
echo-planar imaging pulse sequence. A fast (single-shot) EPI imaging sequence is required i) to
avoid errors caused by bulk motion of the subject and ii) to allow the collection of a large number of
images with different diffusion-gradient directions, for DTI acquisitions, within an acceptable total
scan time.
DT =
D
xx
D
xy
D
xz
D
yx
D
yy
D
yz
D
zx
D
zy
D
zz
A streamline is detected where there the change in
direction of the principle diffusion vector is small
between neighbouring pixels. In the brain this
would indicate a probable white matter tract
Medical Applications of MRI 2011 handout 4
37
position position
Region of
magnetic
field
gradient
Beyond Conventional MRI Contrast Mechanisms
3. BOLD contrast and fMRI
Blood Oxygenation Level Dependant (BOLD) contrast
Deoxygenated haemoglobin is paramagnetic (i.e. has unpaired electrons) - and therefore has a
relatively high magnetic susceptibility.
Oxygenated haemoglobin is diamagnetic - with only a relatively low magnetic susceptibility (close to
that of brain tissue)
Therefore the magnetic susceptibility of blood depends upon the relative proportions of oxy- and
deoxy-haemoglobin it contains and therefore to the blood oxygenation level
The presence of paramagnetic deoxyhaemoglobin in blood vessels causes the magnetic field within
the vessels to be higher than in the surrounding tissue, resulting in local magnetic field gradients,
and hence a reduction in T
2
*
in the tissue:
Thus T
2
*
-weighted images, e.g. gradient-echo based EPI, can be used to detect changes in the local
blood oxygenation.
Functional Magnetic Resonance Imaging (fMRI)
fMRI is primarily applied to studies of the brain.
In the brain, specific "processing" functions are located in well defined anatomic regions, e.g. the
visual cortex, auditory cortex or motor cortex.
During "functional activation", as a result of increased metabolic demand, these regions demonstrate
focally increased blood flow.
B
tissue tissue capillary
B
Low oxygenation
-> more deoxyhaemoglobin
-> blood has high magnetic susceptibility
-> high magnetic field gradients around vessels
-> more rapid spin dephasing => short T
2
*
high oxygenation
-> less deoxyhaemoglobin
-> blood has low magnetic susceptibility
-> smaller magnetic field gradients around vessels
-> less rapid spin dephasing => long T
2
*
Medical Applications of MRI 2011 handout 4
38
Time (seconds)
Sudden stimulus
e.g. sound,
flashing light etc,
The haemodynamic response of the brain produces an apparent "over-supply" of blood to the
activated region. Blood volume and blood flow increases, and although more oxygen is being
consumed, the net result is a decrease in the concentration of deoxyhaemoglobin. This causes the
magnetic susceptibility of the blood to decrease. Local microscopic field gradients are reduced and
T
2
*
in the tissue is lengthened. Therefore on T
2
*-weighted images, activated regions demonstrate
increased intensity, or "light up":
Typically a gradient-echo EPI sequence is used in fMRI so that images of the whole brain may be
obtained every few seconds.
Changes in the local value of T2* due to neuronal activation can therefore be rapidly followed,
allowing the location of specific functional areas to be determined to improve understanding of how
the brain performs certain tasks.
T
2
*-weighted
Signal
intensity
Deoxy-
haemog
lobin
Blood
flow
Metabolic
demand
At rest:
On activation:
Signal
intensity
Blood
flow
Metabolic
demand
T
2
*
0 2 3 6 8 10 12
Blood
suscepti
bility
T
2
*
Bold Response to a stimulus
Medical Applications of MRI 2011 handout 4
39
Regional activation detected with BOLD contrast can be demonstrated e.g. in the visual cortex (in
response to patterns and lights), in the motor cortex (in response to finger movement) and in the
noun and verb mediation speech centres of the brain responsible for forming responses to word
presentation tasks.
fMRI is becoming clinically useful for planning neurosurgical procedures where it is important to
know where certain critical functional regions (e.g. speech centres) are located so that they may be
avoided during the surgical approach.
Practical Issues
a) A high temporal resolution is required and therefore EPI is usually used, so the spatial resolution is
limited (for this reason, for display purposes, fMRI data are frequently overlaid upon high resolution
anatomical reference images)
b) The absolute signal change is dependent upon experimental parameters, so is not a direct
measure of blood flow, c.f. PET.
c) The signal changes upon activation are small (< 5%) so averaging between experiments in the same
subject, and even across subjects, is often performed
d) Complex image processing and statistical analysis is required.
-----------------------------------------------------------------------------------------------------------------
Contrast Agents in MRI
Contrast agents provide additional image contrast in order to improve diagnosis yielding:
- increased sensitivity
- increased specificity
- functional information
MRI contrast agents themselves are not seen directly in MRI images, but their presence causes
changes in the relaxation times of the surrounding tissue water.
Paramagnetic contrast agents are commonly used; they contain a metal ion having unpaired
electrons. The most efficient elements are:
Element Symbol Number of unpaired electrons
Gadolidium Gd
3+
7
Manganese Mn
2+
5
Dysprosium Dy
3+
5
Iron Fe
2+
4
Medical Applications of MRI 2011 handout 4
40
These ions are toxic, and are therefore bound in stable, biochemically-inert complexes (known as
chelates) e.g. DTPA (diethylene-triamine-penta-acetic acid) which form low molecular weight water-
soluble agents which are in general safely excreted by the kidneys in a few hours.
Gadolidium compounds are commonly used (high paramagnetic moment) e.g. Gd-DTPA (e.g.
"Magnevist"):
Such contrast agents may affect the MRI signal in 2 ways:
1) Positive Contrast Effect
As the paramagnetic molecules tumble due to thermal motion, they produce local random rotating
magnetic fields in the vicinity of the water protons. If these time-varying magnetic fields have
components at the correct frequency (e.g. the Larmor frequency) they interact with tissue water
spins causing a shortening of the T
1
and T
2
relaxation times. Under normal imaging conditions, the
dominant effect is T
1
shortening and regions taking up the agent appear bright (positive contrast) on
T
1
-weighted imaging sequences such as progressive saturation.
After injection these agents distribute into the intravascular and extracellular space of the body, but
the relatively large molecules cannot cross the intact blood-brain barrier (BBB). Pathological
breakdown or absence of the BBB allows contrast agents to cross into the extracellular space of the
brain and alter T
1
values locally.
Pathologies in which this occurs include tumours, infarctions, infection and acute demyelination. In
cancer sometimes living tumour tissue can be distinguished from the necrotic core and surrounding
oedema in tissue outside the tumour.
Dynamic studies can be used to assess organ function, such as filtration rate in the kidneys, liver
function or membrane permeability in the brain.
Medical Applications of MRI 2011 handout 4
41
2) Negative Contrast Effect
In healthy brain these types of contrast agents are restricted to the blood vessels and cant pass
directly into the brain tissue. They make the blood more paramagnetic and hence microscopic field
gradients are produced around the blood vessels (capillaries) causing a reduction in T
2
*. Therefore
signal intensity in T
2
*
-weighted images is reduced as the contrast agent passes through the tissue
(this is very similar to T2* shortening in BOLD contrast).
This effect can be used to assess local blood flow through the brain. If a bolus of agent is injected into
a vein it will eventually pass through the brain via the heart. If rapid T
2
*-weighted images are
repeatedly acquired (using an EPI sequence to produce an image every second or so) we can track
the passage of the contrast agent bolus through the brain as a function of time:
Such signal intensity curves provide information about tissue perfusion.
Cerebral perfusion is a general term used to describe the delivery of oxygen and other metabolic
substrates to the brain tissue via the blood. It is measured in terms of the Cerebral Blood Flow (CBF).
CBF quantifies the rate of blood supply via the capillaries to a unit mass of brain tissue. It is non-
directional and is approximately 100ml/100g/min in healthy brain. If CBF decreases to less than
approx 20 ml/100g/min for any substantial period, cell death will occur. This might occur acutely
following the rupture or blockage of a blood vessel (i.e. a stroke) or chronically after long-term
disruption of the normal blood supply to the brain. Assessment of perfusion using MRI contrast
agents in this way is therefore an important clinical tool.
By analyzing the shape of the curve of T2*-weighted signal intensity against time as the bolus of
contrast agent passes through the brain (e.g. the duration, width and depth of the signal dip) it is
possible estimate CBF directly.
From shape of curve (time-to-
minimum, overall time-integral
etc) perfusion can be quantified
Time (seconds)
0 5 10 15 20 25
T2*-weighted
image
intensity
Bolus
Injection
Medical Applications of MRI 2011 handout 4
42
The Safety of Gadolinium Contrast Agents
Until recently gadolinium MRI contrast agents were considered relatively safe. However, in 2006
several studies suggested an association between the disease nephrogenic systemic fibrosis (NSF)
and MRI contrast agent administration. NSF is an uncommon but serious acquired systemic disorder
which affects patients with impaired kidney function. NSF causes swelling and tightening of the skin,
usually limited to the extremities, but 5% or less of patients have exceedingly rapid disease course
that may result in death.
It is hypothesised that NSF may be caused by the gadolinium ion becoming dechelated, i.e. separated
from the chelate complex, allowing the toxic ion to circulate unshielded in the blood stream.
Therefore before gadolinium contrast agents are administered, a blood test is usually performed to
ensure that the patients kidney function is adequate to allow the contrast agent to be excreted
sufficiently rapidly, before dechelation can occur.
We also now prefer to use contrast agents with a cyclic molecular structure in preference to those
with a linear structure (such as Magnevist) as these form chelates which are considered to be more
stable.
Other Contrast Agents
Other types of contrast agent are in use or under development, many of them organ-specific
targeting e.g. the liver, specific tumours and the heart. Some, such as magnetite, a
Time
S.I.
Low perfusion e.g. cerebral white matter
High perfusion (e.g. cerebral grey
matter)
Gadolinium Contrast Agents Summary
positive contrast: T
1
-weighted progressive saturation imaging
Shortened T1 signal increases
negative contrast: T
2
*-weighted (gradient-echo) imaging
Shortened T2* signal decreases
Medical Applications of MRI 2011 handout 4
43
superparamagnetic particle which can be coated with an inert resin, may be taken orally (as well as
intravenously), enhancing image contrast in the gut.
Exciting recent developments include the development of smart MRI contrast agents, which
produce paramagnetic contrast only if certain specific important biochemical/molecular species are
expressed in tissue, and iron-oxide agents which may be used to magnetically label macrophages
(and also, potentially, stem cells), so that their distribution within tissue may be tracked.
Medical Applications of MRI 2010 handout 5
- 44 -
Magnetic Resonance Angiography
Angiography is the direct visualization of flowing blood in arteries and veins.
We will consider 2 methods of magnetic resonance angiography (MRA):
(i) Time-of-flight (TOF) MRA
(ii) Phase-contrast (PC) MRA
Both generate contrast caused by the movement (flow) of blood, and both are "bright-blood"
methods where blood vessels appear bright compared with background tissue.
Time-of-Flight Angiography
A gradient echo imaging sequence is used with short repetition time (TR << T
1
) and high flip angle (o
= 40-90
o
) producing a very strong T1 weighting. Due to incomplete relaxation a reduced equilibrium
signal is established for static tissue which appears dark, i.e. the background tissue with relatively
long T
1
produces a low signal similarly to the T1-weighted progressive saturation sequence discussed
earlier.
With the timing shown between each o
o
excitation pulse a fresh volume of blood flows into the
imaging slice. This blood has experienced no previous RF pulses, its magnetization is fully relaxed
(equal to that of M
0
for blood), and therefore gives a comparatively strong signal ( M
o
blood)
Imaging
Slice
v
M
z
Blood
rapid recovery due
to inflow
M
z
o
o
o
o
o
o
o
o
T
R
T
R
Short
(<< T
1
tissue)
Low signal from
static tissue
Slow recovery due
to T
1
relaxation
Artery
(blood flow
velocity, v)
Static tissue
(v = 0)
High signal from
flowing blood
M
z
tissue
Medical Applications of MRI 2010 handout 5
- 45 -
compared with static tissue. Thus arteries containing rapidly flowing blood appear bright, and the
static tissue appears dark.
Phase-Contrast Angiography
Phase-contrast (PC) MRA uses an imaging pulse sequence with additional motion sensitizing
gradients in a manner similar to diffusion-weighted imaging. The difference is that here we are
interested in uniform coherent fluid flow, where all molecules in a pixel move with the same velocity
v, whereas for DWI, the molecules within a pixel move with velocities having random directions and
magnitudes.
PC MRA relies on the creation of a uniform phase shift for all spins within a pixel as the blood moves
in a gradient applied parallel to the direction of flow.
between each o
o
pulse a fresh volume of blood flows into the imaging slice
Imaging
Slice
z
v = 0
v = z/(2T
R
)
v > z/(2T
R
)
image
Some, or all, of the
partially saturated
spins in the blood
vessel are replaced,
increasing the vessel
signal
Partially saturated spins unsaturated spins
Medical Applications of MRI 2010 handout 5
- 46 -
After a RF excitation pulse, spins precess (rotate) in the rotating frame about the z axis, and gain a
phase | in a time t:
The degree of velocity encoding is defined in terms of the velocity encoding factor, or venc:
Frequency, e = .B phase, | = } e dt
= .G
x.
.x(t) =} .B dt
= .G
x.
.(x
0
+ v.t)
For static spins: x = x
0
For flowing spins: x = x
o
+ v.t
After the application of velocity sensitizing gradients:
Phase, |(2T) =
}
T
dt
2
0
=
T
T
x x
T
x x
t v G t x G t v G t x G
2
2
0
0
2
0
. . .
2
1
. . . . . .
2
1
. . .
(
+
(
+
= - . G
x
. v.T
2
G
x
-G
x
Equal magnitude and durations
STATIC SPINS
FLOWING SPINS
P
h
a
s
e
,
|
P
h
a
s
e
,
|
time
time
time
x
y
0
T 2T
|(2T) = 0
A|(2T) = -.G
x
.v.T
2
A| is a phase increment due to
blood velocity
A|
Velocity sensitizing
gradients
Medical Applications of MRI 2010 handout 5
- 47 -
Venc = /GT
2
(units are m.s
-1
)
The venc represents the maximum velocity which can be encoded unambiguously, i.e. the
velocity which produces a A| of 180
o
.
PC MRA is usually performed using a gradient echo sequence. Scanner imperfections (e.g. B0 non-
uniformity) can cause phase differences between neighbouring pixels which have nothing to do with
blood flow. In order to cancel out these unwanted phase differences, in the simplest form of PC
MRA, the image is acquired twice: once with velocity encoding gradients applied in a positive
direction, and once with the same velocity encoding gradients applied in a negative direction. It is
possible to reconstruct each MRI image such that a value for the phase of the MR signal in each pixel
is obtained. For each pixel, the phase angle obtained with the positive velocity encoding gradients
applied is subtracted from the phase angle obtained for the same image pixel acquired with negative
flow-sensitization gradients. For static tissue, the phase is the same in each case and subtraction
results in a phase value of zero. However, for pixels containing flowing blood there is a phase
difference between each image (proportional to the flow velocity) and the final subtraction image, in
which the pixel intensity is proportional to the phase difference, shows only flowing blood.
In order to produce images without distracting signal from outside the tissue (i.e. from air) where the
signal phase is random, the phase subtraction image is usually multiplied, pixel-by-pixel, with the
conventional magnitude image.
Occasionally, instead of calculating phase differences for each pixel, PC MRA data is processed by
treating the signal from each pixel as a complex number (i.e. the x axis corresponds to the real axis
and y to the imaginary axis), and performing a complex subtraction between the two images for each
pixel. This can provide better flow-contrast in certain circumstances.
PC MRA has the advantages that it may be made sensitive to vessels containing slowly flowing blood
(the signal from which is likely to be completely suppressed in the TOF technique), and by judicious
choice of the velocity encoding gradients, flow velocity and direction may be estimated. A
disadvantage of PC MRA compared with TOF MRA is that at least 2 acquisitions must be performed
(one with and one without velocity encoding) and so imaging times are longer
MRA image processing/presentation
For both methods, 3-dimensional data sets are acquired (either by using multi-slicing or true 3D
sequences) and algorithms such as the Maximum Intensity Projection (MIP) method used to highlight
image 1:
(+ve velocity
encoding)
Resultant
vector for
flowing spins
image 2:
(negative velocity
encoding)
-
static spins
Flowing spins
static spins
Flowing spins
image 1 image 2:
(complex subtraction)
=
Resultant for
Static spins is zero
Medical Applications of MRI 2010 handout 5
- 48 -
the 3D vascular structure by selecting only the brightest pixels for display and thereby further
suppressing the static background. In this way interactive display of the 3D distribution of blood
vessels viewed from any direction is possible.
MR angiography can be improved with the use of:
a) contrast agent adminsitatration to shorten blood T
1
and hence increase its signal relative to
tissue (as in contrast enhanced magnetic resonance angiography or CEMRA)
b) an additional MTC off-resonance pre-saturation pulse applied to decrease the intensity of
background signal relative to that of blood.
These values
become pixel
values for first
row of MIP direction
of blood
flow
stationary
tissue
MR
angiogram
Maximum Intensity projection for MRA
maximum
intensity
projection
3D stack
of images
Take series of rays across image:
get maximum value along each ray
one slice
through
3D image-
data set
blood
vessel
Medical Applications of MRI 2011 handout 6
- 44 -
Safety in MRI
MRI does not use ionizing radiation and is therefore generally considered a very safe imaging
modality.
However various factors involved in MRI represent hazards to both patients and staff. These include:
(i) The static magnetic field, B
0
.
(ii) Time varying magnetic fields i.e. the switched imaging gradients
(iii) The radio frequency (B
1
) fields.
(iv) The cryogens (liquid nitrogen and liquid helium) required to maintain the
superconductivity of the magnet
(v) Intravenous contrast agents (see discussion in earlier lecture)
Safety guidelines for the management of these risks are laid down (in the UK) by the Department of
Health (via the MHRA) (DB 2007(03) Safety Guidelines for Magnetic Resonance Imaging Equipment
in Clinical Use
http://www.mhra.gov.uk/Publications/Safetyguidance/DeviceBulletins/CON2033018 )
Hazards due to the MRI scanner environment
These are principally hazards created by the static B
0
magnetic field which extends beyond the
confines of the scanner over an ellipsoidal region centred on the centre of the magnet. The stray
fields of whole-body MRI systems may extend over a number of metres.
To reduce the stray fields, 2 types of shielding are used:
1. Passive Shielding iron plates are attached either to the outside of the cryostat, or within
the walls, floor and ceiling of the scanner room
2. Active Shielding The superconducting coil winding is continued in the opposite direction
outside the inner main magnet winding. This self-shielding partially cancels the field
outside the main magnet coils thereby reducing the magnetic field exterior to the scanner.
The safety guidelines state that the general public should not be exposed to fields of more than 5
Gauss (0.5 mT) since cardiac pacemakers and other active medical implants may be affected above
this field strength.
A Controlled Area is defined to enclose the 5 Gauss field contour lines. Signs and physical barriers
must be used to restrict access to areas inside the 5 Gauss contour line.
Projectiles: The most imminent danger to patients and personnel is from ferromagnetic objects such
as pens, scissors etc. which may be attracted to the magnet with great force and act as projectiles.
Ferrous objects experience a displacement force if the field is varying in space, and also a rotational
torque even if the magnetic field is uniform.
All staff and patients are required to empty their pockets and remove jewellery etc. before
approaching the scanner. Access to the scanner room is restricted by locked doors to prevent the
Medical Applications of MRI 2011 handout 6
- 45 -
unauthorized introduction of ferromagnetic material. Metal detectors may be used at the entrance of
scanner suites to reduce this hazard.
Items introduced into the Controlled Area should be correctly labelled as
1. MR Safe
an item which poses no known hazards in all MR environments
2. MR Conditional
An item which has been demonstrated to pose no known hazards in a specified MR
environment with specified conditions of use. Field conditions that define the specified MR
environment include field strength, spatial gradient, dB/dt (time rate of change of the
magnetic field), radio frequency (RF) fields, and specific absorption rate (SAR). Additional
conditions, including specific configurations of the item, may be required.
3. MR Unsafe
an item which is known to pose hazards in all MR environments
Implants: surgical implants e.g. surgical clips, pins, plates, prostheses, neurostimulators, implanted
infusion pumps or pacemakers pose a danger: implants can shift position due to magnetic forces with
a risk of haemorrhage or other injury. They may also get dangerously hot during scanning, and active
electronic implants may malfunction.
Established implants such as pins and plates attached to bones should not move, and teeth fillings
and false teeth are usually not affected.
Patient notes are examined and patients asked to complete a safety questionnaire before scan. A
particular danger may be posed from any particles of metal dust in eyes: often a plane x-ray may be
required to exclude this possibility.
Pacemakers: These may be affected by fields of 17 Gauss or more, and for safety a 5 Gauss threshold
is specified for public exposure (e.g. in corridors surrounding the scanner). Patients with pacemakers
should not be scanned (although MRI Conditional pacemakers are now coming on to the market).
Patient notes are examined and patients asked to complete a safety questionnaire before scan.
Cooling gases (cryogens): In superconducting magnets a "quench" (a very rare event) involves the
magnet windings loosing their superconductivity and becoming resistive, when due to the large
current they carry they heat up rapidly causing all the liquid helium and nitrogen in the magnet to
evaporate very rapidly: large volumes of gas are produced which may completely fill the scanner
suite.
Because of the risks of asphyxia, provision is made to vent these gases to the outside via suitable
piping, and oxygen sensors trigger emergency extraction systems if the fraction of air in the scanner
room drops below safe levels.
Bio-effects: Hazards due to electromagnetic field interactions during scanning
Exposure to MRI is considered safe for patients and staff`. There is no evidence of the initiation of
cancer or other harmful effects.
Medical Applications of MRI 2011 handout 6
- 46 -
Static magnetic fields (B
0
): There is no evidence of risk due to short or long term exposure. Ethical
approval is required to scan human subjects at magnetic fields greater than 4Tesla. Research MRI
scanners use fields up to 8 Tesla for human studies.
Time varying magnetic fields (G
x
, G
y
, G
z
imaging gradients): As the magnetic field gradients are
switched on and off during scanning, the resulting fluctuating magnetic fields (dB/dt) can induce
electrical currents in tissue which may exceed the nerve depolarization threshold and cause
peripheral nerve stimulation (PNS). While this unlikely to cause permanent injury, it may cause
discomfort to patients or cause them to move ruining the scan. To prevent PNS, safety guidelines
specify that dB/dt should not exceed 20T/s for times greater than 120 microseconds.
Software checks and hardware interlocks prevent the scanner from accidentally exceeding this dB/dt
limit.
(Between 2 and 5 T/s, magnetic stimulation of the optic nerve or retina can occur, producing a
harmless flashing sensation in the eyes (magnetic phosphenes)).
Gradient switching acoustic noise: A second hazard resulting from the switched imaging gradients
concerns acoustic noise. This arises due to movement of the gradient coil windings against their
mountings caused by the Lorenz force arising as the current through them is rapidly changed in the
presence of the strong static magnetic field. This acoustic noise may be stressful for patients or even
hazardous. Noise levels increase with field strength and gradient switching speed.
Hearing protection (ear plugs or ear defenders) is commonly provided.
Radio frequency (B
1
) fields: Rapidly oscillating RF electro-magnetic fields can induce currents in
electrically conductive tissue of sufficient magnitude to cause significant heating. The higher the
frequency of the RF used (i.e. the higher the magnetic field strength), the larger the amount of heat
deposited in the tissue by a given RF pulse. Tissues with a high concentration of ions will absorb the
greatest RF power. Parts of the body with a low blood supply are at greatest risk (e.g. the eyes). The
specific absorption rate (SAR), measured in watts per kilogram, is used to quantify the rate of tissue
heating. For a particular imaging sequence the SAR depends upon the field strength, the number of
RF pulses per unit time, their magnitude, the RF coil design and the mass of tissue exposed. Safety
guidelines state that in normal uncontrolled operation the whole-body SAR should not exceed 2
W/kg for exposure times up to 15 minutes. The guidelines are designed to limit the temperature rise
of the tissue to less than 1
0
C.
MRI systems have software checks and hardware interlock systems to prevent the SAR guidelines
from being exceeded. In order to accurately estimate the mass of tissue exposed, it is necessary to
know the weight of the patient, and this is therefore entered at the scanner console prior to scanning.
You might also like
- Collection of over 30 MRI books and guidesDocument2 pagesCollection of over 30 MRI books and guidesAnto Oi100% (1)
- Basics of MRI PDFDocument44 pagesBasics of MRI PDFBMT50% (2)
- MRI Safety Guide for Healthcare ProvidersDocument30 pagesMRI Safety Guide for Healthcare ProvidersadhityaNo ratings yet
- Diagnostic Radiology MCQ ReviewDocument7 pagesDiagnostic Radiology MCQ ReviewMuhammad TariqNo ratings yet
- Siemens Mri Mr-Glossary En-02841199Document176 pagesSiemens Mri Mr-Glossary En-02841199Marina Ivosevic Milosavljevic100% (6)
- Magnetic Resonance Imaging: Equations and RelationsDocument6 pagesMagnetic Resonance Imaging: Equations and Relationswalter jesusNo ratings yet
- Bogaert Clinical Cardiac MRI 2nd PDFDocument722 pagesBogaert Clinical Cardiac MRI 2nd PDFtudoranluciana1100% (1)
- Brain Tumour Detection Document (Final)Document57 pagesBrain Tumour Detection Document (Final)aishu aishuNo ratings yet
- (F) MRI Physics With Hardly Any Math (F) MRI Physics With Hardly Any MathDocument62 pages(F) MRI Physics With Hardly Any Math (F) MRI Physics With Hardly Any Mathdrqazi777No ratings yet
- Introduction to MRI: Understanding Magnetic Properties & Relaxation TimesDocument16 pagesIntroduction to MRI: Understanding Magnetic Properties & Relaxation TimesjuhiNo ratings yet
- MRI Bioeffects and SafetyDocument54 pagesMRI Bioeffects and SafetyYuda FhunkshyangNo ratings yet
- Fast Spin Echo: Bioe 594 - Advanced Topics in Mri Nick GruszauskasDocument42 pagesFast Spin Echo: Bioe 594 - Advanced Topics in Mri Nick GruszauskasagithiaNo ratings yet
- Understanding MRI Basic MR Physics For PhysiciansDocument17 pagesUnderstanding MRI Basic MR Physics For PhysiciansRuxandra Badiu100% (1)
- MRI BasicsDocument73 pagesMRI BasicsLyla BrjanNo ratings yet
- Mri BasicsDocument51 pagesMri BasicsChristian Barba YañiquezNo ratings yet
- MRI Parameters and OptionsDocument10 pagesMRI Parameters and Optionsjitendra guptaNo ratings yet
- MRI Safety Guidelines V2Document36 pagesMRI Safety Guidelines V2perapanNo ratings yet
- Fundamentals of MRI - A Brief History and Basic PrinciplesDocument64 pagesFundamentals of MRI - A Brief History and Basic PrinciplesGieDaquiuagNo ratings yet
- Preclinical MRI - Methods and Protocols (PDFDrive)Document454 pagesPreclinical MRI - Methods and Protocols (PDFDrive)Dominus VobiscumNo ratings yet
- Mri Master RodillaDocument9 pagesMri Master RodillaAndrea BelénNo ratings yet
- Magnetic Resonance Imaging: Physical PrinciplesDocument35 pagesMagnetic Resonance Imaging: Physical PrinciplesBERVIN KINGSNo ratings yet
- Basic Pulse SequencesDocument10 pagesBasic Pulse Sequencesjunghs2000No ratings yet
- Mri Sequences: T1-T2: Sarah Humaira Pembimbing: Dr. H. Ali Imran Lubis, Sp. RadDocument26 pagesMri Sequences: T1-T2: Sarah Humaira Pembimbing: Dr. H. Ali Imran Lubis, Sp. RadSarah Humaira Dennison100% (1)
- MRI Safety Study GuideDocument49 pagesMRI Safety Study GuideJeremiah A.No ratings yet
- Summary of BASICS OF DIFFUSION MRI PDFDocument13 pagesSummary of BASICS OF DIFFUSION MRI PDFParamitha AdriyatiNo ratings yet
- MriDocument28 pagesMriaptivaxrayNo ratings yet
- Knee MriDocument32 pagesKnee MriSyahrul RaziNo ratings yet
- Complete Revision Notes FRCRDocument354 pagesComplete Revision Notes FRCREsraa AlmassriNo ratings yet
- MSK MRI Pulse SequencesDocument62 pagesMSK MRI Pulse SequencesOscar NogueraNo ratings yet
- Interpretation of Magnetic Resonance Imaging of Orbit: Simplifi Ed For Ophthalmologists (Part I)Document7 pagesInterpretation of Magnetic Resonance Imaging of Orbit: Simplifi Ed For Ophthalmologists (Part I)Abid AliNo ratings yet
- Radiological Investigations: Mri & PetDocument59 pagesRadiological Investigations: Mri & PetDeeptanu GhoshNo ratings yet
- (Philip T. English DCR, Christine Moore DCR (Auth.Document188 pages(Philip T. English DCR, Christine Moore DCR (Auth.Åri Budianto100% (1)
- Radiology Recent AdvancesDocument66 pagesRadiology Recent AdvancesDr.P.NatarajanNo ratings yet
- MRI Physics Ch12Document53 pagesMRI Physics Ch12麥宗敬No ratings yet
- MRI PhysicsDocument113 pagesMRI PhysicsSaeed Ahmed ShaikhNo ratings yet
- CT PhysicsDocument117 pagesCT PhysicsGarima Bharti100% (2)
- Mri SequencesDocument13 pagesMri SequencesNorma RamirezNo ratings yet
- MR Physics PrimerDocument98 pagesMR Physics PrimerLaurentiu Radoi100% (1)
- Radiology PDFDocument7 pagesRadiology PDFDidimo Antonio Paez ClavijoNo ratings yet
- Mri Artifacts FinalDocument44 pagesMri Artifacts FinalSunny SbaNo ratings yet
- VMARTDocument92 pagesVMARTCyc XimenaNo ratings yet
- MRI ArtifactsDocument62 pagesMRI ArtifactsMohammed Alhatou100% (2)
- Aplication of MRI Contrast AgentDocument26 pagesAplication of MRI Contrast AgentDKpaljrixNo ratings yet
- Radiology Notes (1-36)Document83 pagesRadiology Notes (1-36)el spin artifactNo ratings yet
- MRI Safety Screening GuidlinesDocument5 pagesMRI Safety Screening GuidlinesEmi Murniati100% (1)
- MRI BasicDocument5 pagesMRI BasicMuhammad Qasim Khan100% (1)
- Women's Imaging: MRI with Multimodality CorrelationFrom EverandWomen's Imaging: MRI with Multimodality CorrelationMichele A. BrownRating: 5 out of 5 stars5/5 (1)
- Magnetic Resonance Imaging - Physical and Biological PrinciplesDocument529 pagesMagnetic Resonance Imaging - Physical and Biological PrinciplesThamiris Rosa100% (2)
- Rtog 0813 Marina CousinsDocument22 pagesRtog 0813 Marina Cousinsapi-426094285No ratings yet
- MR Requirements Musculoskeletal MRIDocument56 pagesMR Requirements Musculoskeletal MRIDifa ZafiraNo ratings yet
- Radio 25 E1 Lec 10 MSK RadiologyDocument3 pagesRadio 25 E1 Lec 10 MSK RadiologyYavuz DanisNo ratings yet
- My MRIDocument58 pagesMy MRIapi-26159412100% (1)
- Basics of Diffusion and Perfusion MRIDocument11 pagesBasics of Diffusion and Perfusion MRIPudhiavan AruviNo ratings yet
- Sprawls Magnetic Resonance Imaging PMT PDFDocument184 pagesSprawls Magnetic Resonance Imaging PMT PDFTHE VIRTUAL CLASSROOMNo ratings yet
- MRI Clinical Application 1Document17 pagesMRI Clinical Application 1Alberto AlbertoNo ratings yet
- FRCR Clinical Radiology Lectures: 2 Diagnostic Radiology Fluoroscopy and FluorographyDocument29 pagesFRCR Clinical Radiology Lectures: 2 Diagnostic Radiology Fluoroscopy and FluorographyDr Piyush100% (1)
- Basic Chest RadiologyDocument81 pagesBasic Chest RadiologynasirimbbNo ratings yet
- Philips Learning Center Enewsletter Issue 3Document1 pagePhilips Learning Center Enewsletter Issue 3Suresh NarayanaswamyNo ratings yet
- MRI Artifacts - Detecting Distortions: Devi KrishnaDocument11 pagesMRI Artifacts - Detecting Distortions: Devi KrishnaDiya Krishna100% (1)
- European Guidelines On Quality Criteria For Computed Tomography PDFDocument2 pagesEuropean Guidelines On Quality Criteria For Computed Tomography PDFBennettNo ratings yet
- Ultrasound Basic Understanding and Learning The LanguageDocument9 pagesUltrasound Basic Understanding and Learning The LanguageWijitha VarenniNo ratings yet
- Magnetic Resonance Spectroscopy and Its Clinical Applications: A ReviewDocument21 pagesMagnetic Resonance Spectroscopy and Its Clinical Applications: A Reviewale saenzNo ratings yet
- Siemens MAGNETOM Sonata 1.5T Tech SpecsDocument3 pagesSiemens MAGNETOM Sonata 1.5T Tech SpecsAbood Ali0% (1)
- Principles of Magnetic Resonance ImagingDocument11 pagesPrinciples of Magnetic Resonance ImagingAnonymous DuA3jEqUqNo ratings yet
- MRT User Manual 3.2.3Document31 pagesMRT User Manual 3.2.3Jöy V. LàûdèNo ratings yet
- Mri Srtle .Document40 pagesMri Srtle .falhazmi0069No ratings yet
- Body MRI ArtifactsDocument19 pagesBody MRI ArtifactsCristián Martínez BocazNo ratings yet
- Transmit-Receive Radiofrequency Coil With Individually Shielded ElementsDocument12 pagesTransmit-Receive Radiofrequency Coil With Individually Shielded ElementsMIQINo ratings yet
- Mri Physics en Rev1.3Document76 pagesMri Physics en Rev1.3Arbjan Rusi100% (3)
- Experimental Indications of Non-Classical Brain FunctionsDocument11 pagesExperimental Indications of Non-Classical Brain FunctionsJohnNo ratings yet
- Recover From AdversityDocument20 pagesRecover From AdversityA. AlejandroNo ratings yet
- bk978 1 6817 4068 3ch0Document21 pagesbk978 1 6817 4068 3ch0fysmaNo ratings yet
- Mri AssignmentDocument3 pagesMri AssignmentaparjotNo ratings yet
- ?silent? MRI With Soft Gradient Pulses: Magnetic Resonance in Medicine August 1999Document6 pages?silent? MRI With Soft Gradient Pulses: Magnetic Resonance in Medicine August 1999muzamiNo ratings yet
- Acronimos de Las Secuencias de PulsoDocument8 pagesAcronimos de Las Secuencias de PulsoAlfredo CastroNo ratings yet
- Use of 2.1 MHZ MRI Scanner For Brain Imaging and Its Preliminary ResultsDocument8 pagesUse of 2.1 MHZ MRI Scanner For Brain Imaging and Its Preliminary ResultsMIQINo ratings yet
- Magnetic Resonance Imaging: Physical PrinciplesDocument35 pagesMagnetic Resonance Imaging: Physical PrinciplesBERVIN KINGSNo ratings yet
- Matrix, Gradients & Signals Image Formation & K-Pace: OutlineDocument17 pagesMatrix, Gradients & Signals Image Formation & K-Pace: OutlineJulieta MateosNo ratings yet
- Essential Musculoskeletal MRI - A Primer For The Clinician (PDFDrive)Document270 pagesEssential Musculoskeletal MRI - A Primer For The Clinician (PDFDrive)Thamiris RosaNo ratings yet
- Understanding Medical Imaging TechnologiesDocument9 pagesUnderstanding Medical Imaging TechnologiesSajjala Poojith reddyNo ratings yet
- The Physics of Functional Magnetic Resonance Imaging (fMRI) : Reports On Progress in PhysicsDocument31 pagesThe Physics of Functional Magnetic Resonance Imaging (fMRI) : Reports On Progress in PhysicslupblaNo ratings yet
- MAGNETOM - Free-Max - Special-Issue - Short RSNA Edition 2020Document49 pagesMAGNETOM - Free-Max - Special-Issue - Short RSNA Edition 2020Leonardo Beltran GalvisNo ratings yet
- SternMed Marcom 0.35T LeafletDocument8 pagesSternMed Marcom 0.35T LeafletQCNo ratings yet
- Gradient Echo Imaging: Review: MR Physics For CliniciansDocument16 pagesGradient Echo Imaging: Review: MR Physics For CliniciansFelipe Gustavo Tercero Vega AbantoNo ratings yet
- Mri SequencesDocument13 pagesMri SequencesNorma RamirezNo ratings yet
- Neuroimaging Biomarkers in Alzheimer's DiseaseDocument136 pagesNeuroimaging Biomarkers in Alzheimer's DiseaseInternational Medical PublisherNo ratings yet
- Magnetic Resonance ImagingDocument21 pagesMagnetic Resonance ImagingAron JaroNo ratings yet
- Mri Physics AacDocument196 pagesMri Physics AacAmit Kumar Mudgal100% (1)