Professional Documents
Culture Documents
Antioxidant Activity of Caffeic Acid
Uploaded by
Rebeca CtOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Antioxidant Activity of Caffeic Acid
Uploaded by
Rebeca CtCopyright:
Available Formats
Toxicology 217 (2006) 213220
Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid)
Ilhami G ulcin
Faculty of Arts and Sciences, Department of Chemistry, Atat urk University, TR-25240 Erzurum, Turkey
Received 25 August 2005; received in revised form 27 September 2005; accepted 27 September 2005
Available online 21 October 2005
Abstract
Caffeic acid (3,4-dihydroxycinnamic acid) is among the major hydroxycinnamic acids present in wine; sinapic acid, which is a
potent antioxidant. It has also been identied as one of the active antioxidant. In the present study, the antioxidant properties of
the caffeic acid were evaluated by using different in vitro antioxidant assays such as 2-azino-bis(3-ethylbenzthiazoline-6-sulfonic
acid) (ABTS) radical scavenging, 1,1-diphenyl-2-picryl-hydrazyl free radical (DPPH
) scavenging, total antioxidant activity by
ferric thiocyanate method, total reductive capability using the potassium ferricyanide reduction method, superoxide anion radical
scavenging and metal chelating activities. -Tocopherol, trolox, a water-soluble analogue of tocopherol, butylated hydroxyanisole
(BHA), and butylated hydroxytoluene (BHT) were used as the reference antioxidant compounds. At the concentrations of 10 and
30 g/mL, caffeic acid showed 68.2 and 75.8% inhibition on lipid peroxidation of linoleic acid emulsion, respectively. On the other
hand, 20 g/mL of standard antioxidant such as BHA, BHT, -tocopherol and trolox indicated an inhibition of 74.4, 71.2, 54.7 and
20.1% on peroxidation of linoleic acid emulsion, respectively. In addition, caffeic acid is an effective ABTS
+
scavenging, DPPH
scavenging, superoxide anion radical scavenging, total reducing power and metal chelating on ferrous ions activities.
2005 Elsevier Ireland Ltd. All rights reserved.
Keywords: Caffeic acid; 3,4-Dihydroxycinnamic acid; Antioxidant activity; Metal chelating; Reducing power; Radical scavenging
1. Introduction
Phenolic compounds are secondary plant metabo-
lites and naturally present in almost all plant mate-
rials, including food products of plant origin. These
compounds are thought to be an integral part of both
human and animal diets (Psomiadou and Tsimidou,
2002). Phenolic acids are simple phenols because of
their structure. Hydroxycinnamic acid is the major sub-
group of phenolic compounds (Sanchez-Moreno et al.,
1998; Sroka and Cisowski, 2003). Hydroxycinnamates
are phenylpropanoid metabolites and occur widely in
plants (Herrmann, 1989), and plant products (Clifford,
Tel.: +90 442 2314444; fax: +90 442 2360948.
E-mail addresses: igulcin@atauni.edu.tr, igulcin@yahoo.com.
1999). Hydroxycinnamates and their derivates are bioac-
tive plant food ingredients. They exhibit in vitro antioxi-
dant activity, which might have benecial health impact
in vivo (Kroon and Williamson, 1999).
Caffeic acid (3,4-dihydroxycinnamic acid) has been
shown to be a -tocopherol protectant in low-density
lipoprotein (LDL) (Laranjinha et al., 1995). Also, its
conjugates such as chlorogenic and caftaric acids were
demonstrated to be more powerful antioxidants in a num-
ber of different systems (Meyer et al., 1998; Fukumoto
and Mazza, 2000). Caffeic acid and its derivatives are
good substrates of polyphenol oxidases, and under cer-
tain conditions may undergo oxidation in plant tissues
or products of plant origin (Kerry and Rice-Evans, 1998;
Bassil et al., 2005).
The importance of reactive oxygen species (ROS)
and free radicals has attracted increasing attention over
0300-483X/$ see front matter 2005 Elsevier Ireland Ltd. All rights reserved.
doi:10.1016/j.tox.2005.09.011
214
I. G ul cin / Toxicology 217 (2006) 213220
the past decade. ROS which include free radicals such
as superoxide anion radicals (O
2
), hydroxyl radicals
(OH
) andnon-free radical species suchas H
2
O
2
andsin-
glet oxygen(
1
O
2
), are various forms of activatedoxygen.
These molecules exacerbate factors in cellular injury and
aging process (Halliwell and Gutteridge, 1989; G ulcin
et al., 2002a, 2002b).
ROS is continuously produced during normal physi-
ologic events and they can easily initiate the peroxida-
tion of membrane lipids, leading to the accumulation of
lipid peroxides. However, they are removed by antiox-
idant defence mechanisms. There is a balance between
the generation of ROS and inactivation of ROS by the
antioxidant system in organisms. Under pathological
conditions, ROS is overproduced and results in oxida-
tive stress. ROS is formed when endogenous antioxidant
defence is inadequate. The imbalance between ROS
and antioxidant defence mechanisms leads to oxida-
tive modication in cellular membrane or intracellu-
lar molecules (Duh et al., 1999; B uy ukokuro glu et al.,
2001).
There are a lot of antioxidants that are introduced
to minimize actions of ROS. For example, phenolic
compounds can trap the free radicals directly or scav-
enge them through a series of coupled reactions with
antioxidant enzymes (Rao et al., 1996). According to
recent research, caffeic acid was a superior antioxidant
compared with p-coumaric and ferulic acids, in inhibit-
ing LDL oxidation (Meyer et al., 1998; Cartron et al.,
2001) but also quenching radicals (Kikuzaki et al., 2002)
and singlet oxygen (Foley et al., 1999). Caffeic acid
always behaves as potent antioxidants. In this study,
we evaluated the possible antioxidant effects of caffeic
acid in different in vitro antioxidant assays including
1,1-diphenyl-2-picryl-hydrazyl free radical scavenging,
ABTS radical scavenging, total antioxidant activity by
ferric thiocyanate method, reducing power, superoxide
anion radical scavenging and metal chelating on ferrous
ions activities.
2. Materials and methods
2.1. Chemicals
Caffeic acid (3,4-dihydroxycinnamic acid), nicotinamide
adenine dinucleotide (NADH), BHA, BHT, nitroblue tetra-
zolium (NBT), phenazine methosulphate (PMS), the stable
free radical 1,1-diphenyl-2-picryl-hydrazyl (DPPH
), 3-(2-
pyridyl)-5,6-bis (4-phenyl-sulfonic acid)-1,2,4-triazine (Fer-
rozine), 2,2-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid)
(ABTS), linoleic acid, -tocopherol, polyoxyethylenesorbitan
monolaurate (Tween-20) and trichloroacetic acid (TCA) were
obtained fromSigma (SigmaAldrich GmbH, Sternheim, Ger-
many). Ammonium thiocyanate was purchased from Merck.
All other chemicals used were in analytical grade and obtained
from either Sigma-Aldrich or Merck.
2.2. Total antioxidant activity determination by ferric
thiocyanate method
The antioxidant activity of caffeic acid and standards
was determined according to the ferric thiocyanate method
(Mitsuda et al., 1996). The solution which contains the same
concentration of caffeic acid (1020 g/mL) or standard sam-
ples (20 g/mL) in 2.5 mL of potassium phosphate buffer
(0.04 M, pH7.0) was added to 2.5 mLof linoleic acid emulsion
in potassium phosphate buffer (0.04 M, pH 7.0). Therefore,
5 mL of linoleic acid emulsion contained 17.5 g Tween-20,
15.5 L linoleic acid, and 0.04 M potassium phosphate buffer
(pH 7.0). On the other hand, 5 mL control composed of 2.5 mL
of linoleic acid emulsion and 2.5 mL, 0.04 M potassium phos-
phate buffer (pH7.0). The mixedsolution(5 mL) was incubated
at 37
C in glass ask. The peroxide level was determined
by reading the absorbance at 500 nm in a spectrophotome-
ter (CHEBIOS s.r.l. UV-vis Spectrophotometer) after reaction
with FeCl
2
and thiocyanate (SCN
) at intervals during incuba-
tion. During the linoleic acid oxidation, peroxides are formed
and that leads to oxidation of Fe
2+
to Fe
3+
. The latter ions form
a complex with thiocyanate and this complex has a maximum
absorbance at 500 nm. This step was repeated every 12 h until
the control reached its maximum absorbance value. Therefore,
high absorbance indicates high linoleic acid emulsion oxida-
tion. The solutions without caffeic acid were used as blank
samples. All data on total antioxidant activity are the average
of duplicate experiments. The inhibition percentage of lipid
peroxidation in linoleic acid emulsion was calculated by fol-
lowing equation:
inhibition of lipid peroxidation (%)
= 100 [(A
Control
/A
Sample
) 100
where A
Control
is the absorbance of control reaction and A
Sample
is the absorbance in the presence of caffeic acid sample or
standard compounds (G ulcin et al., 2004a).
2.3. Total reduction capability
The reducing power of caffeic acid was determined by the
method of Oyaizu (1986). Different concentrations of caffeic
acid (1020 g/mL) in 1 mL of distilled water were mixed
with phosphate buffer (2.5 mL, 0.2 M, pH 6.6) and potassium
ferricyanide [K
3
Fe(CN)
6
] (2.5 mL, 1%). The mixture was incu-
bated at 50
C for 20 min. Aliquots (2.5 mL) of trichloroacetic
acid (10%) were added to the mixture. The upper layer of
solution (2.5 mL) was mixed with distilled water (2.5 mL)
and FeCl
3
(0.5 mL, 0.1%), and the absorbance was mea-
sured at 700 nm in a spectrophotometer. Increased absorbance
of the reaction mixture indicates an increase of reduction
capability.
I. G ul cin / Toxicology 217 (2006) 213220 215
2.4. ABTS radical cation decolorization assay
The spectrophotometric analysis of ABTS
+
radical scav-
enging activity was determined according to the method of Re
et al. (1999). The ABTS
+
cation radical was produced by the
reaction between 7 mM ABTS in H
2
O and 2.45 mM potas-
sium persulfate, stored in the dark at room temperature for
12 h. Before usage, the ABTS
+
solution was diluted to get an
absorbance of 0.700 0.025 at 734 nm with phosphate buffer
(0.1 M, pH 7.4). Then, 1 ml of ABTS
+
solution was added
3 mL of caffeic acid solution in ethanol at different concen-
trations (1020 g/mL). After 30 min, the percentage inhibi-
tion at 734 nm was calculated for each concentration relative
to a blank absorbance (ethanol). The ABTS
+
concentration
(mM) in the reaction medium was calculated from the fol-
lowing calibration curve, determined by linear regression (R
2
:
0.9922):
Absorbance = 0.0116 [ABTS
+
] +0.0479
The scavenging capability of ABTS
+
radical was calculated
using the following equation:
ABTS
+
scavenging effect (%)
= [(A
Control
/A
Sample
/A
Control
) 100]
where A
Control
is the initial concentration of the ABTS
+
and A
Sample
is absorbance of the remaining concentration of
ABTS
+
in the presence of caffeic acid.
2.5. 1,1-Diphenyl-2-picryl-hydrazil (DPPH) free radical
scavenging activity
The free radical scavenging activity of caffeic acid was
measured by the 1,1-diphenyl-2-picryl-hydrazil (DPPH
). This
activity was measured by the following methodology described
by Blois (1958) wherein the bleaching rate of a stable free rad-
ical, DPPH
is monitored at a characteristic wavelength in the
presence of the sample. In its radical form, DPPH
absorbs
at 517 nm, but upon reduction by an antioxidant or a radical
species its absorption decreases. Briey, 0.1 mM solution of
DPPH
in ethanol was prepared and 1 ml of this solution was
added to 3 mL of caffeic acid solution in ethanol at different
concentrations (1020 g/mL). After 30 min, the absorbance
was measured at 517 nm. Lower absorbance of the reaction
mixture indicates higher free radical scavenging activity. The
DPPH
concentration (mM) in the reaction mediumwas calcu-
lated fromthe calibration curve determined by linear regression
(R
2
: 0.999):
Absorbance = 9.2872 [DPPH
] +0.097
The capability to scavenge the DPPH
radical was calculated
using the following equation:
DPPH
scavenging effect (%)
= [(A
Control
A
Sample
/A
Control
) 100]
where A
Control
is the absorbance of the control reaction and
A
Sample
is the absorbance in the presence of caffeic acid (G ulcin
et al., 2004c).
2.6. Superoxide anion radical scavenging activity
Measurement of superoxide anion scavenging activity of
caffeic acid was based on the method described by Liu et al.
(1991). Superoxide radicals are generated in PMSNADHsys-
tems by oxidation of NADH and assayed by the reduction of
NBT. In this experiment, the superoxide radicals were gener-
ated in 3 mL of TrisHCl buffer (16 mM, pH 8.0) containing
1 mL of NBT (50 M) solution, 1 mL NADH (78 M) solu-
tion and sample solution of caffeic acid (30 g/mL) in water.
The reaction was started by adding 1 mL of PMS solution
(10 M) to the mixture. The reaction mixture was incubated at
25
C for 5 min and the absorbance at 560 nm was measured
against blank samples. l-Ascorbic acid was used as a con-
trol. Decreased absorbance of the reaction mixture indicates
increased superoxide anion scavenging activity. The inhibition
percentage of superoxide anion generation was calculated by
using the following formula:
%Inhibition = [(A
Control
A
Sample
)/A
Control
] 100
where A
Control
is the absorbance of the l-Ascorbic acid and
A
Sample
the absorbance of caffeic acid or standards (G ulcin et
al., 2004d).
2.7. Ferrous metal ions chelating activity
The chelating of ferrous ions by caffeic acid and standard
molecules was estimated by the method of Dinis et al. (1994).
Briey, caffeic acid (2550 g/mL) in 0.4 mL was added to
a solution of 2 mM FeCl
2
(0.05 mL). The reaction was ini-
tiated by the addition of 5 mM ferrozine (0.2 mL) and total
volume was adjusted to 4 mL with ethanol. Then, the mixture
was shaken vigorously and left at roomtemperature for 10 min.
Absorbance of the solution was measured spectrophotometri-
caly at 562 nm. The inhibition percentage of ferrozineFe
2+
complex formation was calculated by using the formula given
below:
metal chelating effect (%) = [(A
Control
/A
Sample
)/A
Control
]100
where A
Control
is the absorbance of control and A
Sample
the
absorbance in the presence of the caffeic acid or standards.
The control contains FeCl
2
and ferrozine, complex formation
molecules (G ulcin et al., 2004b).
2.8. Statistical analysis
All data on total antioxidant activity are the average of
duplicate analyses. The other analyses were performed in
triplicate. The data were recorded as mean standard devi-
ation and analysed by SPSS (version 11.5 for Windows,
SPSS Inc.). One-way analysis of variance was performed by
ANOVA procedures. Signicant differences between means
216
I. G ul cin / Toxicology 217 (2006) 213220
Fig. 1. Total antioxidant activity of caffeic acid at different concentra-
tions (1020 g/mL), -tocopherol and trolox (30 g/mL).
were determined by Duncans multiple range tests. p-Values
<0.05 were regarded as signicant and p-values <0.01 were
very signicant.
3. Results and discussion
Antioxidants are closely related to their biofunction-
alities, such as the reduction of chronic diseases like
DNA damage, mutagenesis, carcinogenesis and inhi-
bition of pathogenic bacteria growth which is often
associated with the termination of free radical propa-
gation in biological systems (Zhu et al., 2002). Thus,
antioxidant capacity is widely used as a parameter
for medicinal bioactive components. In this study, the
antioxidant activity of the caffeic acid was compared
with BHA, BHT, -tocopherol and its water-soluble ana-
logue trolox. The antioxidant activity of the caffeic acid
has been evaluated in a series of in vitro tests: scav-
enging ABTS
+
and DPPH
, ferric thiocyanate method,
reducing power, scavenging of superoxide anion radical-
generated non-enzymatic system, and metal chelating
activities.
3.1. Total antioxidant activity determination by
ferric thiocyanate method
The ferric thiocyanate method measures the amount
of peroxide produced during the initial stages of oxida-
tion, which is the primary product of oxidation. Total
antioxidant activity of caffeic acid and standard com-
pounds was determined by the ferric thiocyanate method
in the linoleic acid system. Caffeic acid and standards
exhibited effective antioxidant activity. The effects of
various concentrations of caffeic acid (1020 g/mL) on
lipid peroxidation of linoleic acid emulsion are shown in
Fig. 1 and was found to be 68.2 and 75.8%, respectively,
and their activities are close to BHA (74.4%) and BHT
(71.2%), but higher than that of -tocopherol (54.7%)
and trolox (20.1%) at the 20 g/mL concentration.
3.2. Total reductive capability using the potassium
ferricyanide reduction method
In this assay, the yellowcolor of test solution changes
into various shades of green and blue colors depend-
ing on the reducing power of antioxidant samples. The
reducing capacity of a compound may serve as a signif-
icant indicator of its potential antioxidant activity. The
presence of reductants such as antioxidant substances
in the antioxidant samples causes the reduction of the
Fe
3+
/ferricyanide complex to the ferrous form. There-
fore, Fe
2+
can be monitored by measuring the formation
of Perls Prussian blue at 700 nm (Chung et al., 2002).
Fig. 2 depicts the reducing power of the caffeic acid
and standards (BHA, BHT, -tocopherol and trolox)
using the potassium ferricyanide reduction method. For
the measurements of the reductive ability, the Fe
3+
Fe
2+
transformation was investigated in the presence of caf-
feic acid using the method of Oyaizu (1986). The reduc-
ing power of caffeic acid, BHA, BHT, -tocopherol and
trolox were increased with increase of sample concen-
trations. At different concentrations, caffeic acid demon-
strated an effective reducing power (Fig. 2) and these dif-
ferences were statistically signicant (p <0.01). Reduc-
ing power of caffeic acid and standard compounds exhib-
ited the following order: caffeic acid >BHA>BHT>-
tocopherol >trolox.
3.3. ABTS
+
radical scavenging activity
Generation of the ABTSradical cation forms the basis
of one of the spectrophotometric methods that have been
Fig. 2. Total reductive potential of different concentrations
(1020 g/mL) of caffeic acid, BHA, BHT, -tocopherol and trolox
(BHA: butylated hydroxyanisole, BHT: butylated hydroxytoluene).
I. G ul cin / Toxicology 217 (2006) 213220 217
Fig. 3. Scavenging effect of caffeic acid, BHA, BHT, -tocopherol and
trolox on the stable ABTS
+
at different concentrations (1020 g/ml)
(BHA: butylated hydroxyanisole, BHT: butylated hydroxytoluene,
ABTS
+
: 2,2
-azino-bis (3-ethylbenzothiazoline-6-sulfonate) radi-
cals).
applied to the measurement of the total antioxidant activ-
ity of solutions of pure substances, aqueous mixtures
and beverages (Miller, 1996). A more appropriate for-
mat for the assay is a decolorization technique in that
the radical is generated directly in a stable form prior
to reaction with putative antioxidants. The improved
technique for the generation of ABTS
+
described here
involves the direct production of the blue/green ABTS
+
chromophore through the reaction between ABTS and
potassium persulfate.
As seen in Fig. 3, caffeic acid had effective
ABTS
+
radical scavenging activity in a concentration-
dependent manner (1020 g/mL). There is a sig-
nicant decrease (p <0.01) in the concentration of
ABTS
+
due to the scavenging capacity of caffeic
acid. Also, the scavenging effect of caffeic acid and
standards on the ABTS
+
decreased in that order:
caffeic acid >trolox >-tocopherol, which were 92.9,
86.4 and 74.9%, at the concentration of 25 g/mL,
respectively.
3.4. DPPH free radical scavenging activity
Radical scavenging activities are very important due
to the deleterious role of free radicals in foods and in
biological systems. Excessive formation of free radicals
accelerates the oxidation of lipids in foods and decreases
food quality and consumer acceptance (Min, 1998). In
this study, free radical scavenging activities of caffeic
acid and standards such as BHA, BHT, -tocopherol and
trolox were determined using a DPPH method. DPPH
has been widely used to evaluate the free radical scav-
engingeffects of various antioxidant substances (
Ozcelik
et al., 2003).
Fig. 4. Scavenging effect of caffeic acid, BHA, BHT, -tocopherol and
trolox on the stable DPPH
at different concentrations (1020 g/mL)
(DPPH
: 1,1-diphenyl-2-picryl-hydrazyl free radicals, BHA: butylated
hydroxyanisole, BHT: butylated hydroxytoluene).
In the DPPH assay, the antioxidants were able to
reduce the stable radical DPPH to the yellow colored
diphenyl-picrylhydrazine. The method is based on the
reduction of alcoholic DPPH solution in the presence of
a hydrogen-donating antioxidant due to the formation
of the non-radical form DPPH-H by the reaction. With
this method it was possible to determine the antiradi-
cal power of an antioxidant by measuring of a decrease
in the absorbance of DPPH
at 517 nm. Resulting a
color change from purple to yellow, the absorbance
decreased when the DPPH
was scavenged by an antiox-
idant through donation of hydrogen to form a stable
DPPH
molecule. In the radical form, this molecule
had an absorbance at 517 nm which disappeared after
acceptance of an electron or hydrogen radical from an
antioxidant compound to become a stable diamagnetic
molecule (Matth aus, 2002). Fig. 4 illustrates a signi-
cant decrease (p <0.01) in the concentration of DPPH
radical due to the scavenging ability of caffeic acid and
standards. BHA, BHT, -tocopherol and trolox were
used as references for radical scavengers. The scaveng-
ing effect of caffeic acid and standards on the DPPH
radical decreased in the order of BHT>acid >BHA>-
tocopherol >trolox, which were 99.7, 93.9, 86.2, 85.2
and 14.3%, at the concentration of 20 g/mL, respec-
tively. Free radical scavenging activity of these samples
also increased with an increasing concentration.
3.5. Superoxide anions radical scavenging activity
Superoxide anions are a precursor to active free
radicals that have potential of reacting with biologi-
cal macromolecules and thereby inducing tissue dam-
age (Halliwell and Gutteridge, 1984). Also, it has been
implicated in several pathophysiological processes due
218
I. G ul cin / Toxicology 217 (2006) 213220
Fig. 5. Comparisonof superoxide anionradical scavengingandferrous
ions chelating activities of caffeic acid, BHA, BHT, -tocopherol and
trolox at the same concentration (10 g/mL) (BHA: butylated hydrox-
yanisole, BHT: butylated hydroxytoluene).
to its transformation into more reactive species such as
hydroxyl radical that initiate lipid peroxidation. Super-
oxide has also been observed to directly initiate lipid
peroxidation (Wickens, 2001). It has also been reported
that antioxidant properties of some avonoids are effec-
tive mainly via scavenging of superoxide anion radi-
cal (Yen and Duh, 1994). Superoxide anion plays an
important role in the formation of other ROS such as
hydrogen peroxide, hydroxyl radical, and singlet oxy-
gen, which induce oxidative damage in lipids, proteins,
and DNA (Pietta, 2000). In addition, superoxide anion
is an oxygen-centred radical with selective reactivity.
These species are produced by a number of enzyme
systems in autoxidation reactions and by non-enzymatic
electron transfers that univalently reduce molecular oxy-
gen. It can also reduce certain iron complex such as
cytochrome c.
Superoxide anion derived from dissolved oxygen by
PMSNADHcoupling reaction reduces NBTin this sys-
tem. In this method, superoxide anion reduces the yellow
dye (NBT
2+
) to produce the blue formazan which is mea-
sured spectrophotometrically at 560 nm. Antioxidants
are able to inhibit the blue NBT formation (Parejo et
al., 2002). The decrease of absorbance at 560 nm with
antioxidants indicates the consumption of superoxide
anion in the reaction mixture. As shown in Fig. 5, the
inhibition percentage of superoxide radical generation
by 10 g/mL concentration of caffeic acid and stan-
dards, were found similar statistically. As can be seen
in Fig. 5, the percentage inhibition of superoxide anion
radical generation by 10 g/mL concentration of caf-
feic acid was found as 61.9%. On the other hand, at the
same concentration, BHA, BHT, -tocopherol andtrolox
exhibited 76.0, 47.3, 71.4 and 78.2% superoxide anion
radical scavenging activity, respectively.
3.6. Ferrous ions chelating capacity
The production of highly ROS such as superoxide
anion radicals, hydrogen peroxide, and hydroxyl radi-
cals is also catalysed by free iron through HaberWeiss
reaction,
O
2
+H
2
O
2
O
2
+OH
+OH
(Haber and Weiss, 1934). Among the transition metals,
iron is known as the most important lipid oxidation pro-
oxidant due to its high reactivity. The ferrous state of iron
accelerates lipid oxidation by breaking down hydrogen
and lipid peroxides to reactive free radicals via the Fen-
ton reaction,
Fe
2+
+H
2
O
2
Fe
3+
+OH
+OH
Fe
3+
ion also produces radicals from peroxides although
the rate is 10-foldless thanthat of Fe
2+
ion(Miller, 1996).
Fe
2+
ion is the most powerful pro-oxidant among the
various species of metal ions (Halliwell and Gutteridge,
1984). Ferrozine can quantitatively formcomplexes with
Fe
2+
. In the presence of chelating agents, the complex
formation is disrupted, resulting in a decrease in the red
color of the complex. Therefore, measurement of color
reduction allows estimating the metal chelating activity
of the coexisting chelator. Lower absorbance indicates
higher metal chelating activity.
Ferrous ion chelating activities of caffeic acid, BHA,
BHT, -tocopherol and trolox are shown in Fig. 5. In
this assay, caffeic acid is interfered with the forma-
tion of ferrous and ferrozine complex, suggesting that
they have chelating activity and are able to capture fer-
rous ion before ferrozine. As can be seen in Fig. 5,
we suggested that caffeic acid may chelate the ferrous
ions with hydroxyl groups. It was reported that the
compounds with structures containing two or more of
the following functional groups: OH, SH, COOH,
PO
3
H
2
, C O, NR
2
, S and O in a favourable
structurefunction conguration can show metal chela-
tion activity (Lindsay, 1996; Yuan et al., 2005).
The difference between caffeic acid and the control
was statistically signicant (p <0.01). In additon, caf-
feic acid exhibited 53.2% chelation of ferrous ion at
10 g/mL concentration. On the other hand, the per-
centages of metal chelating capacity of 10 g/mL of
BHA, BHT, -tocopherol and trolox were found as 72.1,
64.3, 21.6 and 48.5%, respectively. The metal scaveng-
ing effect of those samples decreased in the order of
BHA>BHT>caffeic acid >trolox >-tocopherol.
Metal chelating capacity was signicant since it
reduced the concentration of the catalysing transition
metal in lipid peroxidation. It was reported that chelating
I. G ul cin / Toxicology 217 (2006) 213220 219
agents are effective as secondary antioxidants because
they reduce the redox potential thereby stabilizing the
oxidized form of the metal ion.
4. Conclusion
Present study has clearly shown that caffeic acid was
an effective antioxidant in different in vitro antioxidant
assays including total antioxidant activity by ferric thio-
cyanate method, reducing power, ABTS
+
scavenging,
DPPH
scavenging, superoxide anion radical scaveng-
ing and metal chelating activitiy when it is compared to
standard antioxidant compounds such as BHA, BHT, -
tocopherol, a natural antioxidant, and trolox which is a
water-soluble analogue of tocopherol.
References
Bassil, D., Makris, D.P., Kefalas, P., 2005. Oxidation of caffeic acid in
the presence of l-cysteine: isolation of 2-S-cysteinylcaffeic acid
and evaluation of its antioxidant properties. Food Res. Int. 38,
395402.
Blois, M.S., 1958. Antioxidant determinations by the use of a stable
free radical. Nature 26, 11991200.
B uy ukokuro glu, M.E., G ulcin,
I., Oktay, M., K ufrevio glu,
O.
I., 2001.
In vitro antioxidant properties of dantrolene sodium. Pharmacol.
Res. 44, 491495.
Cartron, E., Carbonneau, M.A., Fouret, G., Descomps, B., Leger, C.L.,
2001. Specic antioxidant activity of caffeoyl derivatives and other
natural phenolic compounds: LDLprotection against oxidation and
decrease in the proinammatory lysophosphatidylcholine produc-
tion. J. Nat. Prod. 64, 480486.
Chung, Y.C., Chang, C.T., Chao, W.W., Lin, C.F., Chou, S.T., 2002.
Antioxidative activity and safety of the 50%ethanolic extract from
red bean fermented by Bacillus subtilis IMR-NK1. J. Agric. Food
Chem. 50, 24542458.
Clifford, M.N., 1999. Chlorogenic acids and other cinnamates: nature,
occurrence and dietary burden. J. Sci. Food Agric. 79, 362
372.
Dinis, T.C.P., Madeira, V.M.C., Almeida, L.M., 1994. Action of pheno-
lic derivates (acetoaminophen, salycilate, and 5-aminosalycilate)
as inhibitors of membrane lipid peroxidation and as peroxyl radical
scavengers. Arch. Biochem. Biophys. 315, 161169.
Duh, P.D., Tu, Y.Y., Yen, G.C., 1999. Antioxidant activity of water
extract of harng jyur (ChrysanthemummorifoliumRamat). Lebens.
Wiss. Technol. 32, 269277.
Foley, S., Navaratnam, S., McGarvey, D.J., Land, E.J., Truscott,
T.G., Rice-Evans, C.A., 1999. Free Radic. Biol. Med. 26, 1202
1208.
Fukumoto, L.R., Mazza, G., 2000. Assessing antioxidant and proox-
idant activities of phenolic compounds. J. Agric. Food Chem. 48,
35973604.
G ulcin,
I., Beydemir, S ., Alici, H.A., Elmastas, M., B uy ukokuro glu,
M.E., 2004c. In vitro antioxidant properties of morphine. Pharma-
col. Res. 49, 5966.
G ulcin,
I., B uy ukokuro glu, M.E., Oktay, M., K ufrevio glu,
O.
I., 2002a.
On the in vitro antioxidant properties of melatonin. J. Pineal Res.
33, 167171.
G ulcin,
I., K ufrevio glu,
O.
I., Oktay, M., B uy ukokuro glu, M.E., 2004d.
Antioxidant, antimicrobial, antiulcer andanalgesic activities of net-
tle (Urtica dioica L.). J. Ethnopharmacol. 90, 205215.
G ulcin,
I., Oktay, M., K ufrevio glu,
O.
I., Aslan, A., 2002b. Determina-
tion of antioxidant activity of lichen Cetraria islandica (L) Ach. J.
Ethnopharmacol 79, 325329.
G ulcin,
I., S at,
I.G., Beydemir, S ., Elmastas, M., K ufrevio glu,
O.
I.,
2004a. Comparison of antioxidant activity of clove (Eugenia
caryophyllata Thunb) buds and lavender (Lavandula stoechas L.).
Food Chem. 87, 393400.
G ulcin,
I., S at,
I.G., Beydemir, S ., K ufrevio glu,
O.
I., 2004b. Evalua-
tion of the in vitro antioxidant properties of extracts of broccoli
(Brassica oleracea L.). Ital. J. Food Sci. 16, 1730.
Haber, F., Weiss, J., 1934. The catalytic decomposition of hydrogen
peroxide by iron salts. Proc. R. Soc. London, Ser. A 147, 332
351.
Halliwell, B., Gutteridge, J.M., 1989. Free Radicals in Biology and
Medicine. Clarendon Press, Oxford, pp. 2330.
Halliwell, B., Gutteridge, J.M., 1984. Oxygen toxicology, oxygen rad-
icals, transition metals and disease. Biochem. J. 219, 14.
Herrmann, K., 1989. Occurrence and content of hydroxycinnamic and
hydroxybenzoic acid compounds in foods. Crit. Rev. Food Sci.
Nutr. 28, 315347.
Kerry, N., Rice-Evans, C., 1998. Peroxinitrile oxidises catechols to
o-quinones. FEBS Lett. 437, 167171.
Kikuzaki, H., Hisamoto, M., Hirose, K., Akiyama, K., Taniguchi, H.,
2002. Antioxidant properties of ferulic acid and its related com-
pounds. J. Agric. Food Chem. 50, 21612168.
Kroon, P.A., Williamson, G., 1999. Hydroxycinnamates in plants and
food: current and future perspectives. J. Sci. Food Agric. 79,
355361.
Laranjinha, J., Vieira, O., Madeira, V., Almeida, L., 1995. Two related
phenolic antioxidants with opposite effects on vitamin E content
in low density lipoproteins oxidized by ferrylmyoglobin: con-
sumption vs. regeneration. Arch. Biochem. Biophys. 323, 373
381.
Lindsay, R.C., 1996. Food additives. In: Fennema, O.R. (Ed.), Food
Chemistry. Marcel Dekker Inc., New York, pp. 778780 (Chapter
12).
Liu, Q., Zhu, G., Huang, P., 1991. Anti-inammatory, analgesic and
sedative effects of Leontice kiangnanensis. Zhongguo Zhong Yao
Za Zhi 161, 5065.
Matth aus, B., 2002. Antioxidant activity of extracts obtained from
residues of different oilseeds. J. Agric. Food Chem. 50, 3444
3452.
Meyer, A.S., Donovan, J.L., Pearson, D.A., Waterhouse, A.L., Frankel,
E.N., 1998. Fruit hydroxycinnamic acids inhibit low density
lipoprotein oxidation in vitro. J. Agric. Food Chem. 46, 1783
1787.
Miller, D.D., 1996. Mineral. In: Fennema, O.R. (Ed.), Food Chemistry.
Marcel Dekker, New York, pp. 618649.
Min, D.B., 1998. Lipid oxidation of edible oil. In: Akoh, C.C., Min,
D.B. (Eds.), Food Lipids Chemistry, Nutrition, and Biotechnology.
Marcel Dekker, New York, pp. 283296.
Mitsuda, H., Yuasumoto, K., Iwami, K., 1996. Antioxidation action of
indole compounds during the autoxidation of linoleic acid. Eiyo to
Shokuryo 19, 210214.
Oyaizu, M., 1986. Studies on product of browning reaction prepared
from glucose amine. Jpn. J. Nutr. 44, 307315.
Ozcelik, B., Lee, J.H., Min, D.B., 2003. Effects of light, oxygen and pH
on the 2,2-diphenyl-1-picrylhydrazyl (DPPH) method to evaluate
antioxidants. J. Food Sci. 68, 487490.
220
I. G ul cin / Toxicology 217 (2006) 213220
Parejo, I., Viladomat, F., Bastida, J., Rosas-Romero, A., Flerlage, N.,
Burillo, J., Codna, C., 2002. Comparison between the radical
scavenging activity and antioxidant activity of six distilled and
nondistilled Mediterranean herbs and aromatic plants. J. Agric.
Food Chem. 50, 68826890.
Pietta, P.G., 2000. Flavonoids as antioxidants. J. Nat. Prod. 63,
10351042.
Psomiadou, E., Tsimidou, M., 2002. Stability of virgin olive
oil. 1. Autoxidation studies. J. Agric. Food Chem. 50, 716
721.
Rao, M.V., Paliyath, G., Ormrod, D.P., 1996. Ultraviolet-band ozone-
induced biochemical changes in antioxidant enzymes of Arabidop-
sis thaliana. Plant Physiol. 110, 125136.
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., Rice-
Evans, C., 1999. Antioxidant activity applying an improved ABTS
radical cation decolorization assay. Free Radic. Biol. Med. 26,
12311237.
Sanchez-Moreno, C., Larrauri, J.A., Saura-Calixto, F., 1998. A proce-
dure to measure the antiradical efciency of polyphenols. J. Sci.
Food Agric. 76, 270276.
Sroka, Z., Cisowski, W., 2003. Hydrogen peroxide scavenging, antiox-
idant and anti-radical activity of some phenolic acids. Food Chem.
Toxicol. 41, 753758.
Wickens, A.P., 2001. Aging and the free radical theory. Resp. Physiol.
128, 379391.
Yen, G.C., Duh, P.D., 1994. Scavenging effect of methanolic extract
of peanut hulls on free radical and active oxygen species. J. Agric.
Food Chem. 42, 629632.
Yuan, Y.V., Bone, D.E., Carrington, M.F., 2005. Antioxidant activity
of dulse (Palmaria palmata) extract evaluated in vitro. Food Chem.
91, 485494.
Zhu, Q.Y., Hackman, R.M., Ensunsa, J.L., Holt, R.R., Keen, C.L.,
2002. Antioxidative activities of oolong tea. J. Agric. Food Chem.
50, 69296934.
You might also like
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Test Bank For Biochemistry 7th Edition CampbellDocument33 pagesTest Bank For Biochemistry 7th Edition CampbellGeorgeAndersonikwq100% (27)
- April 25, 1953 Nature 737: Molecular Structure of Nucleic Acids A Structure For Deoxyribose Nucleic AcidDocument2 pagesApril 25, 1953 Nature 737: Molecular Structure of Nucleic Acids A Structure For Deoxyribose Nucleic AcidMontaño BrandonNo ratings yet
- Illumina Dye SequencingDocument5 pagesIllumina Dye SequencingNguyen TaNo ratings yet
- 12-4 Gene Regulation and MutationDocument26 pages12-4 Gene Regulation and Mutationapi-342334216No ratings yet
- Alsi Mahima Flaxseed Story in Hindi DR OP VermaDocument154 pagesAlsi Mahima Flaxseed Story in Hindi DR OP Vermamanish sharmaNo ratings yet
- Lehninger Multiple ChoiceDocument13 pagesLehninger Multiple ChoiceHugo DuarteNo ratings yet
- Glycolysis Notes B.pharm 2ND Sem PDFDocument4 pagesGlycolysis Notes B.pharm 2ND Sem PDFBharti sain100% (2)
- Food Biotechnology Lecture 1Document34 pagesFood Biotechnology Lecture 1JusticeNo ratings yet
- 3 MTCHM3 LEC EnzymesDocument8 pages3 MTCHM3 LEC EnzymesMahal BarrosoNo ratings yet
- Product Catalogue ANG B2b.indd MalyDocument42 pagesProduct Catalogue ANG B2b.indd MalyAlex NikolishviliNo ratings yet
- Genetics, Lecture 5, Trascription (Slides)Document63 pagesGenetics, Lecture 5, Trascription (Slides)Ali Al-QudsiNo ratings yet
- Gel Electrophoresis PDFDocument13 pagesGel Electrophoresis PDFagromusicNo ratings yet
- What Are LipoproteinsDocument18 pagesWhat Are Lipoproteinsarsal1cheema-88705No ratings yet
- Plant Hormone ReceptorsDocument12 pagesPlant Hormone ReceptorsJitendra MishraNo ratings yet
- CARBOHYDRATESDocument54 pagesCARBOHYDRATESWanivwa NalweyaNo ratings yet
- Physical Education: Quarter 4 - Module 4b: Other Dance FormsDocument20 pagesPhysical Education: Quarter 4 - Module 4b: Other Dance FormsJulius BayagaNo ratings yet
- Oxidative Stress and Antioxidant DefenseDocument558 pagesOxidative Stress and Antioxidant DefenseCLAUDIANo ratings yet
- MCQ 1. in Transcription,: Type of Question: Content FromDocument2 pagesMCQ 1. in Transcription,: Type of Question: Content FromAlex XanderNo ratings yet
- T-Cells and Cell-Mediated ImmunityDocument10 pagesT-Cells and Cell-Mediated Immunityw5waNo ratings yet
- Respiration in Plants Class 11 Notes CBSE Biology Chapter 14 (PDF)Document12 pagesRespiration in Plants Class 11 Notes CBSE Biology Chapter 14 (PDF)A. celestianNo ratings yet
- CHEM 121 Biochemistry For Nurses Unit 1Document26 pagesCHEM 121 Biochemistry For Nurses Unit 1Aaron Wallace50% (8)
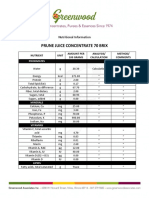
- Prune Juice Concentrate 70 Brix Nutritional InformationDocument2 pagesPrune Juice Concentrate 70 Brix Nutritional Informationborn2dive 9702No ratings yet
- DNA Manipulative EnzymesDocument17 pagesDNA Manipulative EnzymesZain Ul AbedienNo ratings yet
- Varshavsky 1991Document3 pagesVarshavsky 1991kosikevinonuNo ratings yet
- General Biology 1 Module FINALSDocument12 pagesGeneral Biology 1 Module FINALSKenneth Vince AlonzoNo ratings yet
- GLYCOLYSISDocument6 pagesGLYCOLYSISHAZEL SANDRONo ratings yet
- BIOCHEMISTRY BOARD EXAM QUESTIONS-answersDocument7 pagesBIOCHEMISTRY BOARD EXAM QUESTIONS-answerschristinejoan100% (5)
- TestDocument23 pagesTestParth sarthi Sen guptaNo ratings yet
- (YAKEEN 2.0) : Respiration in Plants (Revision Test)Document5 pages(YAKEEN 2.0) : Respiration in Plants (Revision Test)JAGGA GAMING OFFICIALNo ratings yet