Professional Documents
Culture Documents
Current Diagnosis and Management of Retroperitoneal Sarcoma
Uploaded by
Maximiliano Torres0 ratings0% found this document useful (0 votes)
22 views11 pagesRetroperitoneal sarcomas are rare neoplasms that often present with multivisceral involvement. Current data suggest that radical resection remains the optimal chance for cure.
Original Description:
Original Title
177
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentRetroperitoneal sarcomas are rare neoplasms that often present with multivisceral involvement. Current data suggest that radical resection remains the optimal chance for cure.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
22 views11 pagesCurrent Diagnosis and Management of Retroperitoneal Sarcoma
Uploaded by
Maximiliano TorresRetroperitoneal sarcomas are rare neoplasms that often present with multivisceral involvement. Current data suggest that radical resection remains the optimal chance for cure.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 11
July 2011, Vol. 18, No.
3 Cancer Control 177
Introduction
Soft tissue sarcomas are mesenchymal neoplasms that,
when considered in the context of all malignancies, are a
rare entity. Approximately 1% of all newly diagnosed ma-
lignancies are believed to fall in under this classication.
From the Department of Surgery at the University of South Florida
College of Medicine, Tampa, Florida (JEM), and the Sarcoma Pro-
gram at the H. Lee Moftt Cancer Center & Research Institute, Tampa,
Florida (JSZ, RJG).
Submitted August 22, 2010; accepted March 19, 2011.
Address correspondence to Ricardo J. Gonzalez, MD, Sarcoma Pro-
gram, Moftt Cancer Center, 12902 Magnolia Drive, Tampa, FL
33612. E-mail: Ricardo.Gonzalez@moftt.org
No signicant relationship exists between the authors and the
companies/organizations whose products or services may be ref-
erenced in this article.
Jeffrey Hessing. Dawn (Provence). Oil on canvas, 28 36.
Current data suggest that radical
resection remains the optimal
chance for cure for patients with
retroperitoneal sarcoma.
Current Diagnosis and Management of Retroperitoneal Sarcoma
John E. Mullinax, MD, Jonathan S. Zager, MD, and Ricardo J. Gonzalez, MD
Background: Retroperitoneal sarcomas are rare neoplasms that often present with multivisceral involvement.
Treatment for these tumors requires careful decision making requiring a combination of surgery, chemotherapy,
and radiation therapy.
Methods: We reviewed the scientic literature pertaining to the diagnosis and management of retroperitoneal
sarcomas. We also identify recent developments in treatment and discuss future trends in the care of patients
with this disease.
Results: Retroperitoneal tumors often present as large, locally advanced lesions. Evaluation of these tumors
requires careful consideration of a multimodality approach. Retrospective data and historical prospective series
have demonstrated the survival benet of radical resection for these tumors with en bloc resection of involved
structures. Compartmental resections in the retroperitoneum along with debulking of high-grade disease and
regional therapy are controversial approaches with signicant morbidity that can lead to long-term survival.
The application of neoadjuvant and adjuvant therapies in select tumor histologies may improve local control
and survival.
Conclusions: The management of retroperitoneal sarcomas requires a multidisciplinary approach and is best
accomplished at high-volume centers specializing in the care of patients with these complex malignancies. Current
data suggest that radical resection remains the only chance for cure and that chemotherapy and radiation therapy
may confer a survival benet.
There is generally no predominance in terms of gender
or race. Age at presentation is younger compared with
most other malignancies, with many being diagnosed
between 54 to 65 years of age.
1
These tumors can be
found anywhere in the body, with 50% arising in the ex-
tremities, 10% to 15% from the trunk, less than 10% from
the head and neck, and 15% in the retroperitoneum.
1
This manuscript reviews the diagnosis and manage-
ment of retroperitoneal masses, identies recent changes
in the diagnosis and treatment of retroperitoneal sarco-
mas, and discusses future trends in the multidisciplinary
care of patients with this disease. While a comprehensive
review of all that is written from a historical perspective
is not possible here, recent developments in manage-
ment of these patients are reviewed in the context of
evidence-based care.
178 Cancer Control July 2011, Vol. 18, No. 3
Diagnosis
The retroperitoneum offers an environment in which
sarcomas can grow to a large size before they become
symptomatic. Frequently, retroperitoneal sarcomas are
incidentally diagnosed or identied with cross-sectional
imaging as part of a workup for other problems. When
patients do present with symptoms, however, the most
common complaint is abdominal or back pain. Patients
may also present with signs of bowel or ureteral obstruc-
tion due to either extrinsic compression or invasion of
nearby structures. Other common symptoms include
weight loss, anemia, and a palpable abdominal mass.
1
Those aficted may even notice increased abdominal girth
or an uneven contour to their abdominal wall or ank.
Many patients presenting with abdominal complaints
are increasingly evaluated with an imaging modality
shortly after a physical examination. Modern spiral com-
puted tomography (CT) scanners provide an excellent
understanding of the relationship between nearby struc-
tures and are critical to preoperative planning. Given a
patient presenting with a palpable abdominal mass, the
modality of choice should be a high-resolution, thin-cut
CT scan with intravenous and oral contrast.
1-3
The images
allow for further distinction between intra-abdominal and
retroperitoneal structures. Additionally, the difference
between a primary visceral retroperitoneal lesion, retro-
peritoneal adenopathy (possibly from another source),
and a retroperitoneal sarcoma along with involvement
of adjacent viscera may be ascertained. This allows for
an upfront discussion of the need for biopsy if indicated,
the operative plan, and the preparedness of the operative
team, as well as a discussion with the patient regarding
the risks and benets. Magnetic resonance imaging (MRI)
may be useful to aid in the management of lesions in
close proximity to important vascular structures and to
further characterize solid, cystic, necrotic, and enhancing
areas.
2,3
Generally, MRI is used as an adjunct to standard
cross-sectional CT, with oral and intravenous contrast
in select cases. Further discussion of this modality is
discussed later in this manuscript.
Differential Diagnosis
Once a mass in the retroperitoneum is identied, the eti-
ology must be determined. The differential diagnosis in-
cludes a primary neoplasm arising from a retroperitoneal
visceral structure (eg, pancreas, adrenal glands, kidneys,
and duodenum), a retroperitoneal sarcoma, a lymphoma, or
a metastatic lesion. Adenopathy from a malignancy outside
of the retroperitoneum, such as the testicles in a young
male, should be considered. When a mass is discovered,
the patients history becomes paramount to elucidating
a differential diagnosis (Fig 1A-B). When B-symptoms for
lymphoma or a history of scrotal pain or testicular abnor-
mality on physical examination is obtained, attention shifts
away from a primary neoplasm of the retroperitoneum.
Symptoms such as hematuria, uncontrolled hypertension,
or early satiety would be more indicative of a renal, adrenal,
or duodenal primary malignancy, respectively.
Imaging Modalities
Careful consideration of the cross-sectional imaging and
history is key in determining whether a biopsy of the
lesion is indicated. For patients in whom preoperative
systemic therapy or radiation is deemed to be potentially
benecial, a biopsy is mandatory. Aside from patients
receiving preoperative therapy, preoperative CT-guided
biopsy is often utilized at Moftt Cancer Center (MCC)
if the imaging and history are suggestive of a lesion other
than sarcoma or if the lesion has characteristics of an
intermediate- to high-grade retroperitoneal sarcoma such
as large nodules, enhancement, and/or necrosis. If the
lesion is arising from the stomach and may be a gastroin-
testinal stromal tumor, we perform a biopsy of the tumor
using endoscopic ultrasound to avoid the theoretical
risk of seeding the peritoneal cavity.
4,5
We often do not
consider a preoperative biopsy for lesions that appear
to be well-differentiated retroperitoneal liposarcomas as
the sensitivity of CT for low-grade lesions is nearly 100%,
and there is virtually no role for preoperative therapy
for these tumors.
2
Histologic Subtypes
Retroperitoneal sarcomas are mesenchymal neoplasms
that are classied by their histology. They are grouped into
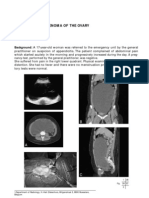
Fig 1A-B. (A) CT demonstrating a retroperitoneal mass (arrow) in a mid-
dle-aged man complaining of abdominal and back pain. Further discussion
revealed occasional night sweats and intermittent scrotal pain. Physical
examination identied a large, indurated right testicle. (B) Transscrotal
ultrasound demonstrated a large, heterogeneous, hypervascular lobular
right testicular mass measuring approximately 7.4 4.4 4.8 cm. He was
subsequently diagnosed with a nonseminomatous testicular cancer and
treated appropriately.
A
B
July 2011, Vol. 18, No. 3 Cancer Control 179
categories based on the characteristics of the mature mes-
enchymal tissues that they resemble. The most common
histology is liposarcoma, followed by leiomyosarcoma.
Other rare subtypes include brosarcoma and malignant
peripheral nerve sheath tumors that are found in the
retroperitoneum. Historically, most soft tissue sarcomas
were classied as malignant brous histiocytomas, but
recent work has demonstrated a signicant proportion of
these tumors are actually dedifferentiated liposarcomas.
1
More exact diagnoses such as this are possible largely
due to recent advances in immunohistochemistry (IHC)
assays. Newer IHC capabilities allow the subtypes to be
more clearly dened based on cell surface markers rather
than histology alone.
6
For the purposes of this review,
we focus on the two common subtypes identied in the
retroperitoneum: liposarcomas and leiomyosarcomas.
Liposarcomas: Liposarcomas are mesenchymal
neoplasms with atypical adipocytes and lipoblasts in a
background of mature adipose tissue. They can appear
anywhere in the body. They represent a spectrum of
tumors with variable behavior that ranges from a low
risk of metastatic potential with well-differentiated tu-
mors to a more aggressive biology with dedifferentiated
lesions. Liposarcomas comprise the most common sub-
type of retroperitoneal sarcomas. Primary retroperito-
neal liposarcomas carry a worse overall prognosis when
compared with liposarcomas that arise from the trunk
and extremities.
1
The discrepancy in survival between
retroperitoneal and nonretroperitoneal primaries can be
explained by the earlier stage and consequent smaller
size at the time of diagnosis for extremity lesions.
1
Liposarcomas can be subdivided into four main his-
tologic subtypes: well-differentiated, dedifferentiated,
myxoid, and round cell.
7
The dedifferentiated category
represents the subtype with a more malignant behavior
and worse overall survival.
7
This aggressive histologic
subtype may present as an initial presentation or may be
found in patients with disease recurrence. Liposarcoma
recurrence is often more difcult to resect with negative
margins due to involvement of adjacent structures and
the development of dedifferentiation.
1
A recent study by Lahat et al
2
examined the utility of
preoperative CT imaging in determining the histologic
classication of liposarcomas as either well-differentiated
or dedifferentiated lesions. They reviewed the images of
78 patients and classied them as either well-differenti-
ated or dedifferentiated based on characteristics such as
their percent of fat, focal nodular/water density, ground
glass opacities, and hypervascularity. Dedifferentiated
lesions were more likely to be inltrative, hypervascular
lesions with areas of necrosis and focal nodular/water
density. Focal nodular/water density was very sensitive
(97.8%) but had a low specicity (51.5%) on nal pathol-
ogy. A radiologic diagnosis of a well-differentiated lesion
was accurate in 100% of patients and as such may be used
as a criterion to avoid preoperative biopsy.
Leiomyosarcomas: These tumors comprise the
second most common histology of retroperitoneal sar-
comas. They can arise anywhere in the body but are
typically more aggressive when found in the retroperi-
toneum, major vascular structures, or intra-abdominal
viscera rather than the skin or subcutaneous tissues. On
histology, these tumors appear as cells in wavy sheets
with cigar-shaped nuclei, and all cells stain positive for
actin on IHC assays.
1
In a review of their collective ex-
perience with leiomyosarcomas, the French Sarcoma
Group found that tumors originating from large vascular
structures accounted for 7% of the total cases of leio-
myosarcomas.
8
They then evaluated the outcomes of
those with tumors of a vascular origin and those with
leiomyosarcomas of a different origin. Based on their
large experience, they found that patients with primary
vascular leiomyosarcomas had a worse median overall
and metastasis-free survival (2.1 years and 0.25 years, re-
spectively) compared to patients with leiomyosarcomas
of other origins (7 years and 9.6 years, respectively).
8
Prognostic Factors
Prior to planning the overall management of retroperito-
neal sarcomas, as with extremity sarcomas, it is important
to reect on the characteristics that are most closely
associated with the prognosis of the disease.
It is well documented that prognosis is dened by
the grade of the sarcoma, regardless of histologic subtype.
In most reports, mitotic activity is the major criterion
used to describe the grade of tumors.
9-13
Not surpris-
ingly, the 5-year survival rate is inversely proportional to
the mitotic activity within these tumors. In an elegantly
designed study led by Singer et al,
14
tumors were divided
into groups based on the number of mitoses per 10 high-
power elds (HPF). Groups consisted of tumors with 0
to 1 mitoses per 10 HPF, those with 1 to 9 mitoses per
10 HPF, and those with > 9 mitoses per 10 HPF. The over-
all survival probability for those with the most mitoses
(32%) was less than those with the least (84%).
After grade as a prognostic factor, the long-term sur-
vival from retroperitoneal sarcomas is most dependent
on the completeness of resection.
7,15,16
While adjuvant
and neoadjuvant therapy is used frequently for these
tumors, complete resection remains the only chance
for long-term survival. Many times, tumor resection in-
volves resection of adjacent viscera with complicated
reconstructions. In all cases, the surgical goal should be
complete resection of the tumor with a microscopically
negative margin (R0). Given the size of these tumors and
their usual proximity to or effacement/invasion of vital
structures, this goal is often difcult to achieve.
In a recent report from MD Anderson Cancer Center
that included a large series of patients, multifocality of
disease was found to be another signicant prognostic
factor.
17
With a large cohort of patients and a median
follow-up of 69 months, the authors found that patients
180 Cancer Control July 2011, Vol. 18, No. 3
presenting with multifocal disease had a signicantly
worse 5-year overall survival rate (31%) compared with
those presenting with unifocal disease (60%). Upon
further examination of the data, they chose a cutoff of
seven lesions as a criterion to determine sarcomatosis.
Multifocal disease restricts the possibility of a complete
resection of all tumors, which is fundamental to long-
term survival with this disease.
Treatment
Role of Surgery
The resectability of a retroperitoneal sarcoma requires
careful study of high-quality preoperative cross-sectional
imaging. Often these tumors will displace nearby struc-
tures; however, more locally advanced or invasive high-
grade primary or recurrent lesions will invade rather than
efface adjacent solid organs, hollow viscera, and major
vascular structures (Fig 2A-C). Given adjacent visceral
or vascular involvement and the likelihood of early dis-
tant metastasis, similar size or smaller adenocarcinomas
would be considered unresectable, but the same is not
generally true for sarcomas.
Some tumors are not amenable to surgical resection
initially. These tumors are typically deemed as such based
on the performance status of the patient and related
comorbidities and the extent of involvement of multiple
areas of bowel where reconstruction is not possible or
will result in malabsorbtion and/or multiple or bilateral
major vascular structures without reasonable proximal
and distal targets for reconstruction, thereby compromis-
ing the viability of critical structures (Fig 3). While these
lesions are often considered unresectable, some benet
may be gained with debulking symptomatic low-grade
lesions in this setting.
18
In some situations an aggressive
operative approach is reasonable if the chance for com-
plete resection is high, particularly with the application
of neoadjuvant therapy in cases where a microscopic
margin-negative resection will be difcult or challeng-
ing. The role of neoadjuvant radiation for retroperitoneal
sarcomas is described later in this review.
In cases of large vessel involvement, the addition of
MRI along with high-quality contrast-enhanced CT scan
can be useful in identifying intraluminal tumor thrombus
and/or the extent of vessel effacement or encasement.
2,3
In general terms, encasement is dened as tumor involve-
ment in > 180 of the vessel and effacement is used when
< 180 of the vessel is involved without caliber change.
While these terms are generally reserved for discussion
of visceral malignancies (eg, pancreatic adenocarcino-
ma), they are also useful in a multidisciplinary discussion
when sarcoma resectability is in question. Generally, a
vessel can be freed from an abutting tumor, but this is
less likely when a mass particularly a high grade le-
sion encases or invades the vessel wall or penetrates
the lumen. This distinction, while having less bearing on
resectability than in visceral malignancies of epithelial
origin, is fundamental to operative planning.
Many times visceral or vascular resection is per-
formed concomitantly with primary tumor extirpation.
Complicated pancreatic and renal en bloc resections
are described throughout the literature.
9,15,19-21
In a re-
cent controversial French study, nearly three-fourths of
Fig 2A-C. CT demonstrating large retroperitoneal dedifferentiated liposar-
coma causing duodenal obstruction. (A) Preoperative CT scan demonstrates
a duodenal obstruction with dilated stomach (arrows). (B) Dedifferentiated
component invades the inferior vena cava (asterisk) and infrarenal aorta
(arrow). Well-differentiated component is indicated by the double asterisk.
(C) Postoperative CT scan 1 year after axillary bifemoral bypass, radical
resection of retroperitoneal tumor with en bloc right nephrectomy, left renal
vein to splenic vein bypass, infrahepatic inferior vena cava resection with
ligation, infrarenal abdominal aorta resection and ligation, pancreatico-
duodenectomy, and right colectomy. The patient continues to show no
evidence of disease.
A
B
C
July 2011, Vol. 18, No. 3 Cancer Control 181
the patients required concomitant visceral resection.
22
Nephrectomy was most commonly performed (42%),
followed by colectomy (30%). The authors advocate for
compartmental resection rather than shelling out of
the tumor and quote a survival difference to support
this claim.
22
It is clear that complete tumor extirpation
is correlated with survival in many studies; however,
with such a radical approach for the retroperitoneum,
the potential for postoperative morbidity is high and
selection bias based on the risk for increased postopera-
tive morbidity limits this approach for all patients with
retroperitoneal sarcomas. Bonvalot et al
22
reported on
their experience with this approach in 374 patients. After
a median follow-up of 4.4 years, 47% of the patients had
recurred locally. Although the authors concluded that
compartmental resection for retroperitoneal sarcomas
was safe, surgical complications developed in 16% of the
patients, with half requiring re-operation. In this study,
13 patients (4%) died in the perioperative setting and 3
died intraoperatively. These numbers are high (though
reportedly acceptable per the authors) and should be
taken into consideration when evaluating this approach
for patients with retroperitoneal sarcomas.
Another European group reported a retrospective
series of patients who underwent visceral en bloc resec-
tions as a part of the primary management of retroperito-
neal sarcomas.
23
Due to a shift toward a more aggressive
surgical approach (en bloc resection of adjacent organs)
during given time periods, patients were divided into two
groups. For patients who underwent surgery during the
earlier time period in which less aggressive resections
were performed, the 5-year local recurrence rate was 48%
compared with 28% for patients treated in the recent
period. It should be noted that there was no guarantee
that patients had en bloc visceral resections in the more
recent group; the patients were merely operated on
during a time period when the institutional preference
was to perform these resections. Additionally, given the
nature of a retrospective review of the two time periods,
the median follow-up was nearly double in the early
group compared to the late group, suggesting that with
longer follow-up, patients in the more radical group tend
to develop recurrences equivalent to patients resected
during the earlier time period.
The issue of compartmental resection in the retro-
peritoneum comes under debate when the operation
entails resection of uninvolved organs. As noted in a
recent editorial,
20
the surgical plan of compartmental
resection as described by Bonvalot et al
22
has some fun-
damental aws. First, this approach advocates radical en
bloc resection of uninvolved nearby structures to obtain
a negative margin; however, selection bias occurs as the
complexity of resection and reconstruction increases
(eg, the spine or aorta). Second, no long-term overall
survival benet has been demonstrated in the patients
who have undergone resection of uninvolved organs.
Others have shown no survival benet in those with
amputation for extremity sarcomas compared to those
with limb preservation therapy. Finally, the data derived
from the study are retrospective and span a long inter-
val; gathering data over a 20-year period provides little
to no control of confounding variables or standardized
criteria for the extent of resection. It is difcult to draw
meaningful conclusions based on patients treated over
such a long period of time.
Although the recent European publications describ-
ing compartmental resection for retroperitoneal sarco-
mas remain controversial, major multivisceral resections
of involved structures are often required to obtain nega-
tive surgical margins. The extent of disease in patients
with retroperitoneal sarcomas can sometimes require
nephrectomy at the time of primary resection. In one
large series by Russo et al,
24
nephrectomy was required
in 20% of patients who had undergone resections. Indi-
cations for nephrectomy included gross tumor invasion
or total encasement. The authors concluded that, based
on a cohort of 53 patients requiring nephrectomy, the
5-year overall survival rate was signicantly increased
compared with a cohort of 20 historical controls who
were left with macroscopic disease.
In terms of vascular invasion or in situations where
the tumor arises from a major vascular structure such as
the inferior vena cava or aorta, combined resection with
vascular reconstruction has been described and is safe
when performed at a center with expertise in these com-
plex procedures.
8,19,25
Most commonly described are pa-
tients with leiomyosarcoma arising from the inferior vena
cava (IVC). Management of the IVC after resection is an
area of debate regarding the need for reconstruction. The
options of simple ligation vs conduit reconstruction are
described, but no study to date describes an advantage
of one option over the other. Based on literature reports,
reconstruction of the IVC is not uniformly required, and
Fig 3. Large desmoid tumor not amenable to resection due to involvement
in small bowel mesentery and superior mesenteric artery and vein branches.
Resection would have compromised blood ow to a large portion of the
small bowel and resulted in infarction or short bowel syndrome.
182 Cancer Control July 2011, Vol. 18, No. 3
ligation alone is generally well tolerated with a surpris-
ingly low incidence of transient lower-extremity edema
or other sequelae.
26-28
The most common postoperative
morbidity is lower-extremity edema and, perhaps surpris-
ingly, acute renal failure.
19
Avoiding this reconstruction
prevents such long-term complications as graft infection
and pulmonary embolism.
As with other diseases requiring surgical interven-
tion, the operative treatment of sarcomas is best under-
taken at a dedicated sarcoma center with physicians
specically focused on the care of patients with this
disease.
29
Outcomes in terms of survival, gross tumor re-
section, and postoperative complications are consistently
better at high-volume centers.
30
In fact, should a biopsy
be undertaken, it is advisable that this be performed at
the treatment center by the surgeon who will ultimately
perform the denitive operation. Retroperitoneal sar-
coma is a rare clinical event and, as such, should be man-
aged in centers that treat the bulk of the regional cases.
Role of Chemotherapy
The role of neoadjuvant chemotherapy is not well de-
ned in retroperitoneal sarcomas due to the rarity of the
disease and therefore is often extrapolated from trials
that include extremity sarcoma. Several case series have
reported complications and toxicities, but no denitive
prospective trials have examined survival differences in
those receiving preoperative vs postoperative chemo-
therapy. In terms of patients who do receive preopera-
tive therapy, the degree of tumor necrosis appears to
be a factor that predicts local recurrence and overall
survival.
31
The theoretical advantage of neoadjuvant
chemotherapy focuses on the potential to reduce the
complexity of the proposed operation for the tumors that
respond to systemic therapy. Meric et al
32
described their
experience with this approach in 65 patients. They deter-
mined that in 13% of patients, the scope of the surgery de-
creased with a resultant higher margin-negative resection
rate and better local recurrence-free and overall survival
rates compared with nonresponders. Furthermore, it
appears that those receiving preoperative chemotherapy
have no increase in perioperative morbidity compared
with those who undergo an adjuvant regimen.
27
At
MCC, we prefer to use preoperative chemotherapy
often prior to external beam radiation selectively
for large intermediate- to high-grade lesions that will
likely require nephrectomy. This is often adminis-
tered with the intention of eliciting response in the
primary lesion or potentially resectable metastatic lesions
with combination doxorubicin/ifosfamide.
33
Patients
who may require nephrectomy are able to better tolerate
higher doses of chemotherapy in the preoperative setting
due to the nephrotoxic side effects of the therapy
particularly ifosfamide on the remaining kidney post-
operatively. While no prospective data are available, the
retrospective case series that exist do not support the
universal use of neoadjuvant chemotherapy for retro-
peritoneal sarcomas and, as such, we prefer to select this
approach carefully for patients with biopsy-proven large,
intermediate- to high-grade tumors and in the context of
a multidisciplinary review.
14,34-36
The role of adjuvant chemotherapy has been studied
as well (Table 1).
37-39
Several prospective randomized
trials have demonstrated decreased local recurrence
rates in patients who received adjuvant regimens, but the
effect on overall survival is less clear. A recent Cochrane
review was undertaken to investigate the effect of adju-
vant therapy in a mixed population of sarcoma patients.
37
Upon review of 14 trials, it was determined that local re-
currence and disease-free survival were positively affected
by adjuvant therapy, but the effect on overall survival
could not be determined due to a lack of data. Several
notable international reports of adjuvant chemotherapy
have supported its use in advanced disease. The French
Sarcoma Group recently reported a large retrospective
cohort series of patients with grade 2 and 3 disease.
39
In the nal analysis, metastasis-free survival and overall
survival were both signicantly improved in patients with
grade 3 disease but not grade 2. Frustaci et al
38
reported
even stronger results in the form of a retrospective study.
Table 1. Adjuvant Chemotherapy for Sarcoma
Study
(yr)
No. of
Patients
Therapy Median
Follow-up
(yrs)
Disease-Free Survival Overall Survival
With
chemotherapy
Without
chemotherapy
With
chemotherapy
Without
chemotherapy
Italiano et al
39
(2010)
420 Doxorubicin-based,
various regimens
9 NR NR 58% at 5 yrs 45% at 5 yrs
SMAC
37
(2000)
1,568 Doxorubicin-based,
various regimens
9.4 70% at 10 yrs 60% at 10 yrs 54% at 10 yrs 50% at 10 yrs
Frustaci et al
38
(2001)
104 Doxorubicin +
ifosfamide
4.9 48 mos 16 mos 75 mos
median
46 mos
median
NR = not reported, SMAC = Sarcoma Meta-analysis Collaboration.
July 2011, Vol. 18, No. 3 Cancer Control 183
They found a nearly three-fold increase in the disease-
free survival for patients who received adjuvant therapy.
More importantly, there was an absolute difference of 29
months between the median overall survival of patients
receiving adjuvant chemotherapy and those who did
not.
38
It should be noted that these reports include pa-
tients with soft tissue extremity sarcoma and little data are
available for the routine use of adjuvant chemotherapy in
the subset of patients with retroperitoneal sarcomas. Data
from soft tissue extremity sarcomas are often generalized
to retroperitoneal sarcomas. This is a usual approach be-
cause of the rare incidence of retroperitoneal sarcomas
and the limitations of designing a clinical trial with such
a small and profoundly heterogenous patient population.
For these reasons, we generally do not advocate adjuvant
systemic therapy outside of a protocol or in the setting of
a multidisciplinary review at a sarcoma center.
For patients with overwhelming intra-abdominal
recurrence, or sarcomatosis, the role of hyperthermic
intraperitoneal chemotherapy (HIPEC) has been inves-
tigated. This is typically delivered in the operating room
after a maximal amount of tumor debulking has been
completed. The chemotherapy is delivered via large bore
catheters directly to the temporarily closed peritoneal
cavity. The chemotherapy is typically platinum-based
(eg, cisplatin or oxaliplatin) and always delivered at hy-
perthermic temperatures of about 42
C. Principles of
this therapy are based largely on specic patient selec-
tion. For maximal benet, only patients with peritoneal
disease (not recurrent solid organ) should be selected,
and maximal cytoreductive surgery should be completed
prior to administration of HIPEC. Patients treated outside
of these parameters will have poorer outcomes.
40
Few randomized trials have evaluated HIPEC in pa-
tients with sarcomatosis, and most of these studies have
demonstrated no benet over debulking alone. A French
group recently published a randomized trial with signi-
cant follow-up that showed no improvement in overall
survival for patients following debulking and peritonec-
tomy after receiving HIPEC compared with those who
did not.
41
Local relapse-free and metastasis-free survival
were identical between the groups as well. Interestingly,
the group reported a low incidence of morbidity asso-
ciated with the therapy, which is contradicted in many
other reports.
Remaining reports in the literature are case series,
either described retrospectively or prospectively.
9,42-45
These are single-institution reports that describe sur-
vival outcomes and the toxicity of the operation (Table
2).
9,41-43,45
All reports indicate that cytoreductive surgery
followed by HIPEC is a safe procedure, but the overall
effectiveness cannot be determined due to the lack of
randomized controls. The nal conclusion from each
report is essentially that although the procedure is safe,
the effect on survival cannot be formally determined until
a prospective randomized trial is conducted.
Given the paucity of data and the marginal response
of a select group of patients, HIPEC should, at this time, be
delivered only at centers with signicant experience in
this modality and only in the setting of clinical protocol.
With the recent organization of the American Society of
Peritoneal Surface Malignancies,
46
there will likely be
forthcoming criteria for agents and regimens that will
be tested in a prospective randomized trial.
Role of Radiation Therapy
Although commonly applied for retroperitoneal sarco-
mas in either the neoadjuvant or adjuvant setting, there
is no level I evidence for radiation therapy in the specic
management of retroperitoneal sarcomas, so data from
extremity soft tissue sarcomas are generally extrapolated.
Decisions about dose and timing need to be modied due
to not only the potential for toxicity to nearby structures,
but also the potential effects relating to the anatomic
connes of this space. Specic concerns remain with the
potential for bowel toxicity. In treating soft tissue sarco-
mas, prescribing doses in the range of 60 Gy to 90 Gy is
not uncommon, but the bowel exhibits signicant signs
of toxicity at doses greater than 45 Gy.
1
For this reason,
regimens must be modied.
Fundamentally, the choice for preoperative vs post-
operative radiation therapy must be made with consid-
eration of wound complications as well as the impact
on local recurrence and survival (Table 3).
13,34,47-50
No
study has shown a survival benet for patients receiving
preoperative radiation compared with those receiving
postoperative radiation.
14,34,51
These same studies do
show an increase in postoperative complications, most
often wound infections or dehiscence.
34
With no survival
benet and an increase in postoperative complications,
preoperative radiation therapy should be used in highly
selected patients in the setting of a high-volume, multi-
disciplinary program focusing on sarcoma.
Table 2. Reports of Hyperthermic Intraperitoneal Chemotherapy
Study No. of
Patients
Regimen Median
Follow-up
(mos)
Median
Overall
Survival
(mos)
Berthet et al
9
(1999)
43 Multiple 20 20
Eilber et al
42
(1999)
54 Mitoxantrone 37 18
Rossi et al
45
(2004)
60 Cisplatin/
doxorubicin
28 34
Bonvalot et al
41
(2005)
19 Cisplatin/
doxorubicin
60 29
Lim et al
43
(2007)
28 Cisplatin/
mitoxantrone
NR 5.5
NR = not reported.
184 Cancer Control July 2011, Vol. 18, No. 3
Preoperative radiation therapy has been studied
largely in the setting of pilot studies on an institutional
basis. Reports from large-volume centers focus on the
safety and feasibility of this therapy. A report from the
University of Alabama, the largest report from a single
institution, indicates that dose escalation is important.
52
Patients received a total of 45 Gy, with a boost of 57.5 Gy
to areas predicted as high probability for positive margins.
The authors suggest that higher doses may be possible.
All patients underwent surgical resection nearly all having
a complete macroscopic resection. Another report from
MD Anderson Cancer Center indicated that preoperative
chemotherapy combined with neoadjuvant radiation may
be of benet.
20
In this series of 35 patients, 83% under-
went surgical resection and 90% of those underwent a
complete macroscopic resection. These reports indicate
that it is safe, but no comparative studies exist and thus
long-term survival conclusions are not possible.
At our center, preoperative radiation is used for high-
ly selected cases generally for intermediate- to high-
grade lesions following a discussion with an interdis-
ciplinary team experienced in the management of these
complex cases. Patients with large tumors are typically
given preoperative radiation under the theory that (1)
the gross tumor volume can be accurately addressed only
preoperatively, (2) the neoplasm itself actually protects
the surrounding viscera (generally bowel or kidney) by
displacing these structures, and (3) tumors with a rich
blood supply have a better response to the radiation.
31
We tend not to use adjuvant radiation therapy for these
patients as the exact surgical margins are more difcult
to assess, even with intraoperative marking using surgical
clips, and the collateral damage to the remaining viscera
can be much higher.
Studies have been reported that focus on ancillary
adjuvant treatments. In one such report of brachytherapy
following resection and neoadjuvant radiotherapy, the
authors believe brachytherapy to be generally safe but
advocate its use only to the lower abdomen, given the
high toxicity of delivery to the upper abdomen.
53
An-
other report of intraoperative radiotherapy describes
the modality as safe, with a radiation-related morbidity
of 14%.
54
In both of these reports, the sample size was
small and the follow-up was insufcient to allow for
conclusions regarding the impact on survival. Even with
the use of radical surgery and pre- or postoperative
chemoradiotherapy, approximately half of patients will
have a local recurrence at a median of 45 months.
7
Man-
agement of recurrent sarcoma is difcult and should
warrant referral to a high-volume center.
Recurrent Disease
Local Recurrence
The overwhelming majority of patients will manifest
local recurrence at some point. Management of this
recurrent disease should involve a coordinated plan
of care among medical, radiation, and surgical oncolo-
gists. In the setting of locally recurrent disease, complete
Table 3. Radiation Therapy for Sarcoma
Study
(yr)
No. of
Patients
Therapy Median Follow-up % Local Recurrence Rate % Overall Survival (yrs)
With
XRT
Without
XRT
With
XRT
Without
XRT
Adjuvant Therapy
Pisters et al
47
(1996)
164 Surgery brachytherapy 6.3 yrs 18 31 84
(5)
81
(5)
Yang et al
48
(1998)
141 Chemotherapy EBRT 9.6 yrs 1.5 23.3 75
(10)
74
(10)
Alektiar et al
49
(2008)
41 Surgery + IMRT 35 mos 4.8 NR 64
(5)
NR
Koshy et al
50
(2010)
2,830 Surgery XRT 5 yrs NR NR 73
(3)
63
(3)
Neoadjuvant Therapy
Caudle et al
34
(2007)
14 Neoadjuvant XRT 11 mos 50 N/A 74
(2)
N/A
Kraybill et al
13
RTOG 9514
(2010)
64 eoadjuvant chemotherapy,
XRT + adjuvant chemotherapy
2.57 yrs 10.1 N/A 75.1
(5)
N/A
EBRT = external beam radiation therapy, IMRT = intensity-modulated radiation therapy, NR = not reported, NA = not applicable.
July 2011, Vol. 18, No. 3 Cancer Control 185
operative extirpation should be the goal. Given that
many of those patients will have undergone an extensive
operation for their primary disease, the decision to re-
enter the abdomen should not be taken lightly. Anatomic
planes will be destroyed and, perhaps, due to signicant
reconstructions, uninvolved organs may be displaced
and therefore more easily injured on reoperation. For
these reasons, reoperation for recurrence should be un-
dertaken if there is evidence of progression and when it
is believed that the complete resection of the recurrent
disease can be accomplished safely.
For those in whom it is believed that surgical resec-
tion will not be possible or that an R2 resection is likely,
systemic therapy should be considered and exploration
of the abdomen reserved for palliative intent.
19
Shibata
et al
18
studied the role of incomplete resection of retro-
peritoneal liposarcomas and determined that tumor de-
bulking may be benecial in patients with unresectable
primary disease; however, this benet is marginal at best
for those with unresectable recurrent disease. Further-
more, a recent report by Park et al
55
studied the growth
rate of 105 patients with locally recurrent retroperitoneal
liposarcomas. They concluded that the size of the locally
recurrent disease, primary histologic variant, grade, and
local recurrence growth rate were independent predic-
tors of disease-specic survival. Improved survival was
identied in those patients with a growth rate of less
than 0.9 cm per month.
Metastatic Disease
As with radiation therapy, there is a paucity of data re-
garding resection of metastatic disease for retroperito-
neal sarcoma. Some important points can be drawn
from evaluations of metastasectomy for extremity soft
tissue sarcoma. Blackmon et al
56
recently reported their
results on pulmonary metastasectomy from soft tissue
sarcoma. The authors reported that the 5-year survival
rate in this highly selected group of patients was nearly
doubled, from 13.5% to 35%, in the patients who under-
went resection of pulmonary metastases compared to
those without resection.
The surgical management of hepatic metastases has
been studied in similar fashion. There are several reports
of extended survival in those who undergo resection of
hepatic metastases,
57-59
with a recent study by Marudanay-
agam et al
57
reporting a 32% 5-year overall survival rate in
patients who underwent resection of hepatic metastases.
A review of metastasectomy studies seems to indicate
that the disease-free interval is the strongest predictor
of benet from hepatic metastasectomy. Several studies
have also investigated the resection of noncolorectal
nonneuroendocrine hepatic metastases. In these patients
as well, there is a survival advantage following resection
of the liver lesions.
60
At MCC, we tend to treat patients with a shorter
disease-free interval or multiple sites of metastasis with
standard systemic therapy or a clinical trial. Metastasec-
tomy in this setting may be undertaken after determining
disease response in patients with acceptable performance
status. Patients with a long disease-free interval, oligo-
metastatic disease, and excellent performance status are
more suitable candidates for metastasectomy with curative
intent. Although several centers report a survival advan-
tage after metastasectomy for sarcoma, this approach may
not be advantageous for patients with multiple sites of
disease or a short disease-free interval. Careful patient
selection in this setting is critical to minimize treatment-
related complications and improve outcomes.
Palliative Procedures
Given the proximity of the primary lesion to bowel
and other viscera, it is not surprising that patients with
unresectable recurrent disease or widely disseminated
disease will require a palliative operation at some point.
The Memorial Sloan-Kettering experience found that a
majority of palliative operations were for gastrointestinal
complaints.
61
More than half of the patients had initial
relief of their symptoms at 30 days, but the effect was
short-lived. When operating on patients with palliative in-
tent, the axiom of rst do no harm should be paramount.
Conclusions
The presentation and the extent of disease in a patient
with retroperitoneal sarcoma are perhaps more varied
than in patients with any other clinical entity. Further-
more, the rarity of retroperitoneal sarcomas makes or-
ganizing appropriately powered prespective studies dif-
cult. Many reports are retrospective, single-institution
reports based on historical controls. Problems encoun-
tered with these studies include the varied clinical ap-
proaches over a time period with a rapidly changing clini-
cal milieu. Larger collaborative prospective controlled
studies among sarcoma centers need to be conducted in
order to establish more powerful conclusions regarding
the management of these complex neoplasms.
The treatment of retroperitoneal sarcomas is based
on surgical resection. All diagnostic algorithms and other
treatment modalities should be managed by a multidisci-
plinary team at a high-volume center that focuses on the
care of patients with sarcoma. The decision for preopera-
tive therapy chemotherapy or radiation should be
based on optimizing the patient for surgical resection.
Therapies that do not enhance the tness of the patient
or the likelihood of complete gross tumor resection will
not improve long-term survival.
References
1. Liles JS, Tzeng CW, Short JJ, et al. Retroperitoneal and intra-ab-
dominal sarcoma. Curr Probl Surg. 2009;46(6):445-503.
2. Lahat G, Madewell JE, Anaya DA, et al. Computed tomography
scan-driven selection of treatment for retroperitoneal liposarcoma histologic
subtypes. Cancer. 2009;115(5):1081-1090.
3. Raut CP, Pisters PW. Retroperitoneal sarcomas: combined-modality
treatment approaches. J Surg Oncol. 2006;94(1):81-87.
186 Cancer Control July 2011, Vol. 18, No. 3
4. Demetri GD, Benjamin RS, Blanke CD, et al. NCCN Task Force re-
port: management of patients with gastrointestinal stromal tumor (GIST)
update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw.
2007;5(suppl 2):S1-S29; quiz S30.
5. Demetri GD, Delaney T; NCCN Sarcoma Practice Guidelines Panel.
NCCN: Sarcoma. Cancer Control. 2001;8(6 suppl 2):94-101.
6. Fabre-Guillevin E, Coindre JM, Somerhausen Nde S, et al. Retro-
peritoneal liposarcomas: follow-up analysis of dedifferentiation after clinico-
pathologic reexamination of 86 liposarcomas and malignant brous histiocy-
tomas. Cancer. 2006;106(12):2725-2733.
7. Singer S, Antonescu CR, Riedel E, et al. Histologic subtype and mar-
gin of resection predict pattern of recurrence and survival for retroperitoneal
liposarcoma. Ann Surg. 2003;238(3):358-370; discussion 370-371.
8. Italiano A, Toulmonde M, Stoeckle E, et al. Clinical outcome of leio-
myosarcomas of vascular origin: comparison with leiomyosarcomas of other
origin. Ann Oncol. 2010;21(9):1915-1921.
9. Berthet B, Sugarbaker TA, Chang D, et al. Quantitative methodolo-
gies for selection of patients with recurrent abdominopelvic sarcoma for
treatment. Eur J Cancer. 1999;35(3):413-419.
10. De Pas T, Toffalorio F, Colombo P, et al. Brief report: activity of imatinib
in a patient with platelet-derived-growth-factor receptor positive malignant
solitary brous tumor of the pleura. J Thorac Oncol. 2008;3(8):938-941.
11. Heslin MJ, Lewis JJ, Nadler E, et al. Prognostic factors associated
with long-term survival for retroperitoneal sarcoma: implications for man-
agement. J Clin Oncol. 1997;15(8):2832-2839.
12. Huang HY, Brennan MF, Singer S, et al. Distant metastasis in retro-
peritoneal dedifferentiated liposarcoma is rare and rapidly fatal: a clinico-
pathological study with emphasis on the low-grade myxobrosarcoma-like
pattern as an early sign of dedifferentiation. Mod Pathol. 2005;18(7):976-984.
13. Kraybill WG, Harris J, Spiro IJ, et al. Phase II study of neoadju-
vant chemotherapy and radiation therapy in the management of high-risk,
high-grade, soft tissue sarcomas of the extremities and body wall: Radiation
Therapy Oncology Group Trial 9514. J Clin Oncol. 2006;24(4):619-625.
14. Singer S, Corson JM, Demetri GD, et al. Prognostic factors predic-
tive of survival for truncal and retroperitoneal soft-tissue sarcoma. Ann Surg.
1995;221(2):185-195.
15. Dalal KM, Kattan MW, Antonescu CR, et al. Subtype specic prog-
nostic nomogram for patients with primary liposarcoma of the retroperito-
neum, extremity, or trunk. Ann Surg. 2006;244(3):381-391.
16. Lahat G, Dhuka AR, Lahat S, et al. Outcome of locally recurrent and
metastatic angiosarcoma. Ann Surg Oncol. 2009;16(9):2502-2509.
17. Anaya DA, Lahat G, Liu J, et al. Multifocality in retroperitoneal sar-
coma: a prognostic factor critical to surgical decision-making. Ann Surg.
2009;249(1):137-142.
18. Shibata D, Lewis JJ, Leung DH, et al. Is there a role for incomplete
resection in the management of retroperitoneal liposarcomas? J Am Coll
Surg. 2001;193(4):373-379.
19. Daylami R, Amiri A, Goldsmith B, et al. Inferior vena cava leiomyo-
sarcoma: is reconstruction necessary after resection? J Am Coll Surg.
2010;210(2):185-190.
20. Pisters PW, Ballo MT, Fenstermacher MJ, et al. Phase I trial of pre-
operative concurrent doxorubicin and radiation therapy, surgical resection,
and intraoperative electron-beam radiation therapy for patients with localized
retroperitoneal sarcoma. J Clin Oncol. 2003;21(16):3092-3097.
21. Gieschen HL, Spiro IJ, Suit HD, et al. Long-term results of intraop-
erative electron beam radiotherapy for primary and recurrent retroperitoneal
soft tissue sarcoma. Int J Radiat Oncol Biol Phys. 2001;50(1):127-131.
22. Bonvalot S, Rivoire M, Castaing M, et al. Primary retroperitoneal
sarcomas: a multivariate analysis of surgical factors associated with local
control. J Clin Oncol. 2009;27(1):31-37.
23. Gronchi A, Lo Vullo S, Fiore M, et al. Aggressive surgical policies in a
retrospectively reviewed single-institution case series of retroperitoneal soft
tissue sarcoma patients. J Clin Oncol. 2009;27(1):24-30.
24. Russo P, Kim Y, Ravindran S, et al. Nephrectomy during operative
management of retroperitoneal sarcoma. Ann Surg Oncol. 1997;4(5):421-424.
25. Stauffer JA, Fakhre GP, Dougherty MK, et al. Pancreatic and mul-
tiorgan resection with inferior vena cava reconstruction for retroperitoneal
leiomyosarcoma. World J Surg Oncol. 2009;7:3.
26. Dew J, Hansen K, Hammon J, et al. Leiomyosarcoma of the in-
ferior vena cava: surgical management and clinical results. Am Surg.
2005;71(6):497-501.
27. Hollenbeck ST, Grobmyer SR, Kent KC, et al. Surgical treatment and
outcomes of patients with primary inferior vena cava leiomyosarcoma. J Am
Coll Surg. 2003;197(4):575-579.
28. Mingoli A, Feldhaus RJ, Cavallaro A, et al. Leiomyosarcoma of the
inferior vena cava: analysis and search of world literature on 141 patients
and report of three new cases. J Vasc Surg. 1991;14(5):688-699.
29. Gutierrez JC, Perez EA, Moffat FL, et al. Should soft tissue sarco-
mas be treated at high-volume centers? An analysis of 4205 patients. Ann
Surg. 2007;245(6):952-958.
30. Lewis JJ, Leung D, Woodruff JM, et al. Retroperitoneal soft-tissue
sarcoma: analysis of 500 patients treated and followed at a single institution.
Ann Surg. 1998;228(3):355-365.
31. Eilber FC, Rosen G, Eckardt J, et al. Treatment-induced pathologic
necrosis: a predictor of local recurrence and survival in patients receiving
neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin
Oncol. 2001;19(13):3203-3209.
32. Meric F, Hess KR, Varma DG, et al. Radiographic response to neo-
adjuvant chemotherapy is a predictor of local control and survival in soft
tissue sarcomas. Cancer. 2002;95(5):1120-1126.
33. Verma S, Younus J, Stys-Norman D, et al. Meta-analysis of ifos-
famide-based combination chemotherapy in advanced soft tissue sarcoma.
Cancer Treat Rev. 2008;34(4):339-347.
34. Caudle AS, Tepper JE, Calvo BF, et al. Complications associated
with neoadjuvant radiotherapy in the multidisciplinary treatment of retroperi-
toneal sarcomas. Ann Surg Oncol. 2007;14(2):577-582.
35. Meric F, Milas M, Hunt KK, et al. Impact of neoadjuvant chemo-
therapy on postoperative morbidity in soft tissue sarcomas. J Clin Oncol.
2000;18(19):3378-3383.
36. Windham TC, Pisters PW. Retroperitoneal sarcomas. Cancer Con-
trol. 2005;12(1):36-43.
37. Sarcoma Meta-analysis Collaboration. Adjuvant chemotherapy for
localised resectable soft tissue sarcoma in adults. Sarcoma Meta-analysis
Collaboration (SMAC). Cochrane Database Syst Rev. 2000;(2):CD001419.
Update in: Cochrane Database Syst Rev. 2000;(4):CD001419.
38. Frustaci S, Gherlinzoni F, De Paoli A, et al. Adjuvant chemotherapy
for adult soft tissue sarcomas of the extremities and girdles: results of the
Italian randomized cooperative trial. J Clin Oncol. 2001;19(5):1238-1247.
39. Italiano A, Delva F, Mathoulin-Pelissier S, et al. Effect of adjuvant
chemotherapy on survival in FNCLCC grade 3 soft tissue sarcomas: a
multivariate analysis of the French Sarcoma Group Database. Ann Oncol.
2010;21(12):2436-2441.
40. Sugarbaker PH. Intraperitoneal chemotherapy and cytoreductive
surgery for the prevention and treatment of peritoneal carcinomatosis and
sarcomatosis. Semin Surg Oncol. 1998;14(3):254-261.
41. Bonvalot S, Cavalcanti A, Le Pchoux C, et al. Randomized trial
of cytoreduction followed by intraperitoneal chemotherapy versus cytore-
duction alone in patients with peritoneal sarcomatosis. Eur J Surg Oncol.
2005;31(8):917-923.
42. Eilber FC, Rosen G, Forscher C, et al. Surgical resection and intraper-
itoneal chemotherapy for recurrent abdominal sarcomas. Ann Surg Oncol.
1999;6(7):645-650.
43. Lim SJ, Cormier JN, Feig BW, et al. Toxicity and outcomes associated
with surgical cytoreduction and hyperthermic intraperitoneal chemotherapy
(HIPEC) for patients with sarcomatosis. Ann Surg Oncol. 2007;14(8):2309-
2318.
44. Rossi CR, Foletto M, Mocellin S, et al. Hyperthermic intraoperative
intraperitoneal chemotherapy with cisplatin and doxorubicin in patients who
undergo cytoreductive surgery for peritoneal carcinomatosis and sarcoma-
tosis: phase I study. Cancer. 2002;94(2):492-499.
45. Rossi CR, Deraco M, De Simone M, et al. Hyperthermic intraperito-
neal intraoperative chemotherapy after cytoreductive surgery for the treat-
ment of abdominal sarcomatosis: clinical outcome and prognostic factors in
60 consecutive patients. Cancer. 2004;100(9):1943-1950.
46. Esquivel J, Stojadinovic A, Levine EA. The American Society of Peri-
toneal Surface Malignancies (ASPSM). Ann Surg Oncol. 2010 Nov 11.
Epub ahead of print.
47. Pisters PW, Harrison LB, Leung DH, et al. Long-term results of a pro-
spective randomized trial of adjuvant brachytherapy in soft tissue sarcoma.
J Clin Oncol. 1996;14(3):859-868.
48. Yang JC, Chang AE, Baker AR, et al. Randomized prospective study
of the benet of adjuvant radiation therapy in the treatment of soft tissue
sarcomas of the extremity. J Clin Oncol. 1998;16(1):197-203.
49. Alektiar KM, Brennan MF, Healey JH, et al. Impact of intensity-mod-
ulated radiation therapy on local control in primary soft-tissue sarcoma of the
extremity. J Clin Oncol. 2008;26(20):3440-3444.
50. Koshy M, Rich SE, Mohiuddin MM. Improved survival with radia-
tion therapy in high-grade soft tissue sarcomas of the extremities: a SEER
analysis. Int J Radiat Oncol Biol Phys. 2010;77(1):203-209.
51. Ballo MT, Zagars GK, Pollock RE, et al. Retroperitoneal soft tissue
sarcoma: an analysis of radiation and surgical treatment. Int J Radiat Oncol
Biol Phys. 2007;67(1):158-163.
52. Tzeng CW, Fiveash JB, Popple RA, et al. Preoperative radiation
therapy with selective dose escalation to the margin at risk for retroperitoneal
sarcoma. Cancer. 2006;107(2):371-379.
53. Jones JJ, Catton CN, OSullivan B, et al. Initial results of a trial of pre-
operative external-beam radiation therapy and postoperative brachytherapy
for retroperitoneal sarcoma. Ann Surg Oncol. 2002;9(4):346-354.
54. Yoon SS, Chen YL, Kirsch DG, et al. Proton-beam, intensity-mod-
ulated, and/or intraoperative electron radiation therapy combined with ag-
gressive anterior surgical resection for retroperitoneal sarcomas. Ann Surg
Oncol. 2010;17(6):1515-1529.
55. Park JO, Qin LX, Prete FP, et al. Predicting outcome by growth rate
of locally recurrent retroperitoneal liposarcoma: the one centimeter per
month rule. Ann Surg. 2009;250(6):977-982.
56. Blackmon SH, Nipam S, Roth J, et al. Resection of pulmonary and
July 2011, Vol. 18, No. 3 Cancer Control 187
extrapulmonary sarcomatous metastases is associated with long-term sur-
vival. Ann Thorac Surg. 2009;88(3):877-885.
57. Marudanayagam R, Sandhu B, Perera MT, et al. Liver resection for
metastatic soft tissue sarcoma: an analysis of prognostic factors. Eur J Surg
Oncol. 2011;37(1):87-92.
58. Chen H, Pruitt A, Nicol TL, et al. Complete hepatic resection of
metastases from leiomyosarcoma prolongs survival. J Gastrointest Surg.
1998;2(2):151-155.
59. Rehders A, Peiper M, Stoecklein NH, et al. Hepatic metastasectomy
for soft-tissue sarcomas: is it justied? World J Surg. 2009;33(1):111-117.
60. Reddy SK, Barbas AS, Marroquin CE, et al. Resection of non-
colorectal nonneuroendocrine liver metastases: a comparative analysis. J
Am Coll Surg. 2007;204(3):372-382.
61. Yeh JJ, Singer S, Brennan MF, et al. Effectiveness of palliative pro-
cedures for intra-abdominal sarcomas. Ann Surg Oncol. 2005;12(12):1084-
1089.
You might also like
- Α Α Α Α-Amylase-Eps: Biosystems S.ADocument1 pageΑ Α Α Α-Amylase-Eps: Biosystems S.ARisqon Anjahiranda AdiputraNo ratings yet
- Benign Retroperitoneal Schwannoma A Case Series and Review of The LiteratureDocument4 pagesBenign Retroperitoneal Schwannoma A Case Series and Review of The LiteratureafmzzqrhardloaNo ratings yet
- Primary Peritoneal Mesothelioma Case Series and Literature ReviewDocument7 pagesPrimary Peritoneal Mesothelioma Case Series and Literature ReviewfvhgssfmNo ratings yet
- Retro Peritoneal TumorDocument37 pagesRetro Peritoneal TumorHafizur RashidNo ratings yet
- Retroperitoneal Tumor - NcbiDocument6 pagesRetroperitoneal Tumor - NcbiWidya Eka WardhaniNo ratings yet
- Bladder NCCNDocument17 pagesBladder NCCNJoriza TamayoNo ratings yet
- Mucoepidermoidcarcinoma of The Pancreas: A Case ReportDocument3 pagesMucoepidermoidcarcinoma of The Pancreas: A Case ReportIJAR JOURNALNo ratings yet
- A Rare Huge Myxofibrosarcoma of Chest WallDocument3 pagesA Rare Huge Myxofibrosarcoma of Chest WallIOSRjournalNo ratings yet
- Principles Of Managing Soft Tissue SarcomasDocument33 pagesPrinciples Of Managing Soft Tissue Sarcomasbashiruaminu100% (1)
- 48 Devadass EtalDocument4 pages48 Devadass EtaleditorijmrhsNo ratings yet
- Initial Diagnosis and Staging of Pancreatic Cancer Including Main DifferentialsDocument33 pagesInitial Diagnosis and Staging of Pancreatic Cancer Including Main DifferentialsClaudia YalanNo ratings yet
- Fabrega 2016Document12 pagesFabrega 2016Nikos SerifisNo ratings yet
- Overview of The Management of Rectal Adenocarcinoma - UpToDate PDFDocument24 pagesOverview of The Management of Rectal Adenocarcinoma - UpToDate PDFRaíla SoaresNo ratings yet
- TangDocument8 pagesTangPatricia BezneaNo ratings yet
- Small Renal Mass: Clinical PracticeDocument11 pagesSmall Renal Mass: Clinical PracticeHarlina NurlitaNo ratings yet
- View ContentDocument24 pagesView ContentDian AyuNo ratings yet
- Solitary Fibrous Tumor in The Retroperit 2024 International Journal of SurgeDocument5 pagesSolitary Fibrous Tumor in The Retroperit 2024 International Journal of SurgeRonald QuezadaNo ratings yet
- cLINICAL PRACTICE GUIDELINESDocument7 pagescLINICAL PRACTICE GUIDELINESdrmolinammNo ratings yet
- Breast Cancer Mrker NatureDocument12 pagesBreast Cancer Mrker Naturerajasekaran_mNo ratings yet
- Neuroendocrine Tumors Dr. WarsinggihDocument20 pagesNeuroendocrine Tumors Dr. WarsinggihAndi Eka Putra PerdanaNo ratings yet
- Benign Liver LesionsDocument30 pagesBenign Liver LesionstheintrovNo ratings yet
- Surgical Management of Solid Tumor-4674Document7 pagesSurgical Management of Solid Tumor-4674Theofhila AldelaNo ratings yet
- Gastrointestinal Stromal Tumor FirdausDocument8 pagesGastrointestinal Stromal Tumor FirdausFarizka FirdausNo ratings yet
- Overview of The Management of Primary Colon Cancer - UpToDateDocument30 pagesOverview of The Management of Primary Colon Cancer - UpToDateCaio AmaralNo ratings yet
- Seminars in Radiation Oncology - Vol 33 (1) January 2023 - Bladder CancerDocument90 pagesSeminars in Radiation Oncology - Vol 33 (1) January 2023 - Bladder CancerZuriNo ratings yet
- Surgical Management of Primary and Metastatic Spinal TumorsDocument7 pagesSurgical Management of Primary and Metastatic Spinal TumorskikyfauziasuryaNo ratings yet
- Jurnal Radiologi 2Document21 pagesJurnal Radiologi 2Adelya Dwi AsyifaNo ratings yet
- Malignant Giant Pheochromocytoma Case Report and Literature ReviewDocument8 pagesMalignant Giant Pheochromocytoma Case Report and Literature ReviewannesasoumyanNo ratings yet
- 45657Document28 pages45657zoxpop1980No ratings yet
- Differentiating Cystic Liver Lesions: A Review of Imaging Modalities, Diagnosis and ManagementDocument9 pagesDifferentiating Cystic Liver Lesions: A Review of Imaging Modalities, Diagnosis and ManagementMayerlin CalvacheNo ratings yet
- Clinical Profile of Patients Undergoing PancreaticoduodenectomyDocument25 pagesClinical Profile of Patients Undergoing PancreaticoduodenectomyRoscelie KhoNo ratings yet
- Junk For Junk SiteDocument5 pagesJunk For Junk SitejwthreeNo ratings yet
- Colorectal Carcinoma: Diagnostic, Prognostic, and Molecular FeaturesDocument13 pagesColorectal Carcinoma: Diagnostic, Prognostic, and Molecular FeaturesEdward Arthur IskandarNo ratings yet
- Rare Case of Aggressive Malignant Phyllodes Tumor with Rapid MetastasisDocument16 pagesRare Case of Aggressive Malignant Phyllodes Tumor with Rapid MetastasisunknownNo ratings yet
- Seth P Lerner, MD Derek Raghavan, MD, PHD, Facp, Fasco Jerome P Richie, MD, Facs Michael E Ross, MD Contributor DisclosuresDocument10 pagesSeth P Lerner, MD Derek Raghavan, MD, PHD, Facp, Fasco Jerome P Richie, MD, Facs Michael E Ross, MD Contributor DisclosuresDiego SalasNo ratings yet
- Carmignani 2003Document8 pagesCarmignani 2003Mario TrejoNo ratings yet
- Urogenital CancersDocument2 pagesUrogenital CancerssdfNo ratings yet
- Case6. ERISGRUPtesticularcancerDocument3 pagesCase6. ERISGRUPtesticularcancerJardee DatsimaNo ratings yet
- Wilm's Tumor Powerpoint MMDocument36 pagesWilm's Tumor Powerpoint MMWai GyiNo ratings yet
- En - 0120 9957 RCG 33 02 00134 PDFDocument10 pagesEn - 0120 9957 RCG 33 02 00134 PDFbernardo04061993No ratings yet
- SEOM Clinical Guideline of Management of Soft-Tissue Sarcoma (2016)Document8 pagesSEOM Clinical Guideline of Management of Soft-Tissue Sarcoma (2016)Bogdan TudorNo ratings yet
- Behranwala 2003Document11 pagesBehranwala 2003Therese Justine BruelNo ratings yet
- Thesis Gastric CancerDocument4 pagesThesis Gastric Cancergj9ggjry100% (2)
- File 1Document4 pagesFile 1Marsya Yulinesia LoppiesNo ratings yet
- МартDocument55 pagesМартLuisAngelPonceTorresNo ratings yet
- Urologic Clinics of North AmericaDocument160 pagesUrologic Clinics of North AmericaHaytham Ibrahim KamelNo ratings yet
- International Journal of Surgery Case ReportsDocument3 pagesInternational Journal of Surgery Case ReportsJuan Eduardo Rios RodriguezNo ratings yet
- Estudio LAACDocument10 pagesEstudio LAACGerardo Heber Delgado ValleNo ratings yet
- Endometrial Cancer Treatment OptionsDocument18 pagesEndometrial Cancer Treatment OptionsKirsten NVNo ratings yet
- BAB I PancreatoblastomaDocument5 pagesBAB I PancreatoblastomaHana YunikoNo ratings yet
- Journalarticle 0185Document2 pagesJournalarticle 0185Mahmood AdelNo ratings yet
- Leijssen 2018Document10 pagesLeijssen 2018Luis Angel Garcia RuizNo ratings yet
- Journal of Surgical Oncology - 2013 - Fortunato - When Mastectomy Is Needed Is The Nipple Sparing Procedure A New StandardDocument6 pagesJournal of Surgical Oncology - 2013 - Fortunato - When Mastectomy Is Needed Is The Nipple Sparing Procedure A New StandardJethro ConcepcionNo ratings yet
- Literature Review On Pancreatic CancerDocument8 pagesLiterature Review On Pancreatic Cancerea86yezd100% (1)
- Advances in Thymic Carcinoma Diagnosis and Treatment: A Review of LiteratureDocument13 pagesAdvances in Thymic Carcinoma Diagnosis and Treatment: A Review of LiteratureEdo Bagus TantyoNo ratings yet
- 009 - The-Perioperative-and-Operative-Management-of - 2023 - Surgical-Oncology-ClinicsDocument17 pages009 - The-Perioperative-and-Operative-Management-of - 2023 - Surgical-Oncology-ClinicsDr-Mohammad Ali-Fayiz Al TamimiNo ratings yet
- Clinical Practice Guidelines: Penile Cancer: ESMO Clinical Practice Guidelines For Diagnosis, Treatment and Follow-UpDocument10 pagesClinical Practice Guidelines: Penile Cancer: ESMO Clinical Practice Guidelines For Diagnosis, Treatment and Follow-Uphypebeast dopeNo ratings yet
- Actualizacion de Reporte de CA Colorectal 2019Document14 pagesActualizacion de Reporte de CA Colorectal 2019Jairo Lino BNo ratings yet
- Omental RhabdoDocument4 pagesOmental RhabdoDaisuke KiritoNo ratings yet
- Colorectal AdenomasDocument11 pagesColorectal AdenomasMatias FlammNo ratings yet
- Breast Disease: Diagnosis and Pathology, Volume 1From EverandBreast Disease: Diagnosis and Pathology, Volume 1Adnan AydinerNo ratings yet
- EUCAST V - 9.0 - Breakpoint - Tables PDFDocument100 pagesEUCAST V - 9.0 - Breakpoint - Tables PDFRicardo Ariel GianeciniNo ratings yet
- Heart Disease PredictionDocument16 pagesHeart Disease PredictionTarannum ShaikNo ratings yet
- Role of Community Health Nurse in Disaster ManagementDocument3 pagesRole of Community Health Nurse in Disaster ManagementyselleamNo ratings yet
- Gram Positive Cocci Bacteriology ChartDocument2 pagesGram Positive Cocci Bacteriology ChartIsabella CeaNo ratings yet
- Food Handler NotesDocument46 pagesFood Handler NotesMohammad pharabiaNo ratings yet
- OCET-2012 Question Paper for M.Sc. BiochemistryDocument504 pagesOCET-2012 Question Paper for M.Sc. BiochemistryrkbaaiNo ratings yet
- A Simple RP-HPLC Method For Simultaneous Analysis of Paracetmol, Nimesulide and Tizanidine Hydrochloride in Pharmaceutical Dosage FormsDocument9 pagesA Simple RP-HPLC Method For Simultaneous Analysis of Paracetmol, Nimesulide and Tizanidine Hydrochloride in Pharmaceutical Dosage FormsHanimi ReddyNo ratings yet
- Frozen Fresh Food As One of The Solutions To Help Isolated Victims of Disaster Get Healthy FoodDocument52 pagesFrozen Fresh Food As One of The Solutions To Help Isolated Victims of Disaster Get Healthy FoodB1Riansyah FakhiratunnisaNo ratings yet
- Diamond Brochure PDFDocument3 pagesDiamond Brochure PDFSumit SinghNo ratings yet
- DRUG AbuseDocument77 pagesDRUG AbuseJmart84No ratings yet
- Anxiety Disorders: Powerpoint Lecture Notes PresentationDocument43 pagesAnxiety Disorders: Powerpoint Lecture Notes PresentationErsido SamuelNo ratings yet
- Lesson Plan Q4 Week1 Support Agronomic Crop WorkDocument6 pagesLesson Plan Q4 Week1 Support Agronomic Crop WorkTeodi Alden TegioNo ratings yet
- 2018-09-01 Men's Fitness UK PDFDocument116 pages2018-09-01 Men's Fitness UK PDFsalik salmanNo ratings yet
- TLE-HE (Dressmaking) : Activity Sheet Quarter 0 - MELC 4Document15 pagesTLE-HE (Dressmaking) : Activity Sheet Quarter 0 - MELC 4Mari PagxNo ratings yet
- Book Reviews: Halophilic MicroorganismsDocument2 pagesBook Reviews: Halophilic MicroorganismsAlejndrNo ratings yet
- NCP Acute PainDocument3 pagesNCP Acute PainSian Grace AsadaNo ratings yet
- Theoretical Framework - GiannaDocument2 pagesTheoretical Framework - GiannaAnne MarielNo ratings yet
- 1000 Laboratory Management of Haemolysed Samples Results of An RCPAQAP Survey - Ms Penny PetinosDocument37 pages1000 Laboratory Management of Haemolysed Samples Results of An RCPAQAP Survey - Ms Penny PetinosPAUL AVELINO CALLUPENo ratings yet
- Veterinary Medicine 7th EditionDocument2 pagesVeterinary Medicine 7th EditionpintuNo ratings yet
- Powders and GranulesDocument18 pagesPowders and GranulesAlaa Salymeh100% (1)
- Pre-Intermediate/Intermediate Reading Text: LongevityDocument2 pagesPre-Intermediate/Intermediate Reading Text: LongevityMaria Vitória Carvalho100% (1)
- Avhad (2020), Comparison of Effectiveness of Chlorine Dioxide Mouthwash and Chlorhexidine Gluconate Mouthwash in Reduction of Oral Viral Load in Patients With COVID-19Document6 pagesAvhad (2020), Comparison of Effectiveness of Chlorine Dioxide Mouthwash and Chlorhexidine Gluconate Mouthwash in Reduction of Oral Viral Load in Patients With COVID-19Phuong ThaoNo ratings yet
- Essential Messages - 2023 ACSDocument16 pagesEssential Messages - 2023 ACSimran karimNo ratings yet
- Galactocele Is A Benign Breast Tumor of The Mammary Gland Which Is More Prevalent During Pregnancy or LactationDocument4 pagesGalactocele Is A Benign Breast Tumor of The Mammary Gland Which Is More Prevalent During Pregnancy or LactationTheQueensafa90No ratings yet
- Nueva Ecija University of Science and Technology: Republic of The Philippines Cabanatuan CityDocument3 pagesNueva Ecija University of Science and Technology: Republic of The Philippines Cabanatuan CityKazuhero LagranaNo ratings yet
- SSC Biology Preivious QuestionsDocument40 pagesSSC Biology Preivious QuestionsPrabhakar ReddyNo ratings yet
- Desiree Anggia LAPAKHIR2021 DrDesiree DosenMuda KedokteranDocument48 pagesDesiree Anggia LAPAKHIR2021 DrDesiree DosenMuda KedokteranWahyu IndraNo ratings yet
- Conscious Sedation Guidelines for DentistsDocument18 pagesConscious Sedation Guidelines for DentistsAnna NgNo ratings yet
- Reglan Indication Side Effects NursingDocument2 pagesReglan Indication Side Effects NursingRem remNo ratings yet