Professional Documents
Culture Documents
Accelerated Publication
Uploaded by
Francisco Antonó Castro WeithOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Accelerated Publication
Uploaded by
Francisco Antonó Castro WeithCopyright:
Available Formats
THE JOURNAL OF BIOLOGICAL CHEMISTRY
Vol. 277, No. 27, Issue of July 5, pp. 2397723980, 2002
2002 by The American Society for Biochemistry and Molecular Biology, Inc.
Printed in U.S.A.
Accelerated Publication
AMP-activated Protein Kinase
Suppresses Protein Synthesis
in Rat Skeletal Muscle through
Down-regulated Mammalian
Target of Rapamycin (mTOR)
Signaling*
Received for publication, March 22, 2002,
and in revised form, May 2, 2002
Published, JBC Papers in Press, May 7, 2002, DOI
10.1074/jbc.C200171200
Douglas R. Bolster, Stephen J. Crozier,
Scot R. Kimball, and Leonard S. Jefferson
AMP-activated protein kinase (AMPK) is viewed as an
energy sensor that acts to modulate glucose uptake and
fatty acid oxidation in skeletal muscle. Given that protein synthesis is a high energy-consuming process, it
may be transiently depressed during cellular energy
stress. Thus, the intent of this investigation was to examine whether AMPK activation modulates the translational control of protein synthesis in skeletal muscle.
Injections of 5-aminoimidazole-4-carboxamide 1--D-ribonucleoside (AICAR) were used to activate AMPK in
male rats. The activity of 1 AMPK remained unchanged
in gastrocnemius muscle from AICAR-treated animals
compared with controls, whereas 2 AMPK activity was
significantly increased (51%). AICAR treatment resulted
in a reduction in protein synthesis to 45% of the control
value. This depression was associated with decreased
activation of protein kinases in the mammalian target of
rapamycin (mTOR) signal transduction pathway as evidenced by reduced phosphorylation of protein kinase B
on Ser473, mTOR on Ser2448, ribosomal protein S6 kinase
on Thr389, and eukaryotic initiation factor eIF4E-binding protein on Thr37. A reduction in eIF4E associated
with eIF4G to 10% of the control value was also noted. In
contrast, eIF2B activity remained unchanged in response to AICAR treatment and therefore would not
appear to contribute to the depression in protein synthesis. This is the first investigation to demonstrate
changes in translation initiation and skeletal muscle
protein synthesis in response to AMPK activation.
Considerable attention has focused on understanding the
role of AMP-activated protein kinase (AMPK)1 in monitoring
* This study was supported in part by National Institutes of Health
Grant DK15658. The costs of publication of this article were defrayed in
part by the payment of page charges. This article must therefore be
hereby marked advertisement in accordance with 18 U.S.C. Section
1734 solely to indicate this fact.
To whom correspondence should be addressed: Dept. of Cellular and
Molecular Physiology, Penn State College of Medicine, H166, 500 University Dr., Hershey, PA 17033. Tel.: 717-531-8566; Fax: 717-531-7667;
E-mail: jjefferson@psu.edu.
1
The abbreviations used are: AMPK, 5-AMP-activated protein
This paper is available on line at http://www.jbc.org
kinase; AICAR, 5-aminoimidazole-4-carboxamide 1--D-ribonucleoside;
eIF, eukaryotic initiation factor; 4E-BP1, translation inhibitor eIF4Ebinding protein; S6K1, ribosomal protein S6 kinase; PKB, protein kinase B; mTOR, mammalian target of rapamycin; Met-tRNAi, methionyl-tRNAi, DTT, dithiothreitol; ZMP, AICAR-5-monophosphate; PDK,
3-phosphoinositide-dependent protein kinase; PP, protein phosphatase.
23977
Downloaded from http://www.jbc.org/ by guest on May 1, 2016
From the Department of Cellular and Molecular
Physiology, The Pennsylvania State University College
of Medicine, Hershey, Pennsylvania 17033
the energy status of the cell and mediating subsequent metabolic events. AMPK has been referred to as an energy-sensing/
signaling protein within the cell that responds to changes in
the ratio of ATP/AMP as well as phosphocreatine/creatine (1,
2). Changes in the cellular energy state activate AMPK
through various mechanisms involving allosteric regulation of
AMPK, activation by an upstream AMPK kinase, and diminished activity of phosphatases (3). AMPK activation increases
glucose uptake and fatty acid oxidation in muscle (4) as well as
up-regulates expression of various metabolic genes (e.g. the
glucose transporter, GLUT4, uncoupling protein-3, and cytochrome c) (57). Consequently AMPK serves as a sensor/modulator of intermediary metabolism by directing cellular events
to increase energy availability and sustain high energy phosphate levels.
Research using in vitro systems has shown that AMPK can be
activated under artificial conditions such as treatment with high
fructose or 2-deoxyglucose, heat shock, and inhibitors of oxidative
phosphorylation (3). Pharmacological use of 5-aminoimidazole-4carboxamide 1--D-ribonucleoside (AICAR) has been commonly
utilized to directly activate AMPK without altering cellular concentrations of ATP, ADP, and AMP (8). Additionally, starvation
and endurance exercise result in increased activity of AMPK in
skeletal muscle (9 11). Exercise alters the adenine nucleotide
ratios and serves as a physiological context for AMPK activation.
Recently specific catalytic isoforms of AMPK (1 and 2) have
been shown to be differentially regulated by exercise intensity
with 2 AMPK exhibiting greater metabolic sensitivity compared
with the 1 isoform (10, 12).
The concept of AMPK acting as an energy sensor suggests
that cellular processes that utilize ATP, and are not vital to
short term survival, are potential control points for regulation
by the protein kinase (13). Thus, a hierarchy may exist for
ensuring sufficient energy availability during an energetic
stress and the anabolic process of protein synthesis may be
diminished to support that dominant function. The acute control of global rates of protein synthesis is predominantly executed at the level of translational initiation with the modulation of various eukaryotic initiation factors (eIFs) (14).
The protein kinase referred to as the mammalian target of
rapamycin (mTOR), which serves as a convergence point for
signaling by growth factors and amino acids to the mRNA
binding step of translation initiation is involved in modulation
of the phosphorylation of the binding protein for the eukaryotic
initiation factor 4E, i.e. 4E-BP1. It also acts to control the
phosphorylation status of the 70-kDa ribosomal protein S6
kinase (S6K1).
Modulation of these translation initiation events allows for
more immediate control of protein synthesis and is responsive
to changes associated with acute metabolic or nutritional alterations. Therefore, the present investigation examined
whether or not activation of AMPK by treatment with AICAR
would depress translational initiation. We hypothesized that
during an apparent cellular energy stress induced by AICAR,
23978
AMPK Down-regulates PKB/mTOR Signaling
increased AMPK activity would diminish translation initiation
and attenuate protein synthesis.
EXPERIMENTAL PROCEDURES
FIG. 1. The effects of AICAR on AMPK 1 and 2 activity. Muscle
homogenates were centrifuged at 14,000 g for 20 min at 4 C. Supernatants (150 g) were subjected to immunoprecipitation with specific
antibodies to the 1 or 2 catalytic subunits of AMPK and BioMag goat
anti-rabbit beads, and AMPK activity was measured in the immunoprecipitate as described under Experimental Procedures (n 6 per
group). , p 0.001 versus control group.
RESULTS
The experimental model utilized in the studies reported
herein is based on the use of the chemical AICAR to artificially
activate AMPK. AICAR is internalized by the cell and subsequently phosphorylated to form an AMP analog, termed ZMP,
that acts as a metabolic activator of both AMPK and AMPK
kinase without altering the adenine nucleotide ratios within
the cell (8). To assess the effectiveness of AICAR treatment in
the present study, the activity of the 1 and 2 isoforms of
AMPK were measured following immunoprecipitation of the
kinase from skeletal muscle of rats administered either AICAR
or vehicle alone 1 h before analysis. As shown in Fig. 1, activation of the AMPK 1 isoform was unchanged in AICARtreated rats compared with rats administered vehicle alone
(control rats). In contrast, 2 AMPK activity was significantly
elevated (p 0.001) in AICAR-treated animals compared with
controls.
To determine whether or not activation of 2 AMPK in response to AICAR treatment was associated with a change in
protein synthesis, in vivo rates of the synthetic process were
measured using the flooding dose technique (15). The results
show that in AICAR-treated rats, protein synthesis in skeletal
muscle was depressed to 55% of the value (p 0.02) observed
in control animals (Fig. 2). To examine potential mechanisms
regulating the reduction in protein synthesis associated with
increased 2 AMPK activity, several key regulatory steps in
translation initiation were investigated.
The regulation of eIF2 is an important event during translation initiation in maintaining global rates of protein synthesis. The first step in translation initiation is the binding of
methionyl-tRNAi to eIF2GTP to form a ternary complex that
subsequently binds to the 40 S ribosomal subunit. Formation of
the ternary complex can be modulated by phosphorylation of
eIF2 on the -subunit by converting it into a competitive inhibitor of eIF2B activity (21). In the present study the phosphorylation state of eIF2 was examined by protein immunoblot analysis using an anti-phosphopeptide antibody that only
recognizes eIF2 when it is phosphorylated on Ser51. The results show that the relative phosphorylation of eIF2 was
reduced to 80% of the control value (p 0.05) in AICAR-treated
rats. Because the activity of eIF2B can be modulated by the
phosphorylation state of eIF2, the guanine nucleotide exchange activity of eIF2B was measured in extracts of muscle
from AICAR-treated and control animals. However, the activity of eIF2B was not significantly different between the two
groups (0.063 0.009 and 0.056 0.008 pmol of GDP exchanged/min, respectively).
Downloaded from http://www.jbc.org/ by guest on May 1, 2016
AnimalsAnimal facilities and the experimental protocol were reviewed and approved by the Institutional Animal Care and Use Committee of The Pennsylvania State University College of Medicine. Male
Sprague-Dawley rats (175 g) were kept on a 12-h light:dark cycle with
food (Harlan-Teklad Rodent Chow, Madison, WI) and water provided
freely.
AICAR InjectionsRats were injected subcutaneously with AICAR
(1 mg/g of body weight) in sterile 0.9% NaCl, or controls were given an
equivalent volume of 0.9% NaCl (n 6 10 animals per group). A
flooding dose (1.0 ml/100 g body of weight) of L-[2,3,4,5,6-3H]phenylalanine (150 mmol/liter) was injected via the tail vein 50 min after the
subcutaneous injections for the measurement of rates of synthesis of
total mixed muscle protein (15). Rats were sacrificed by decapitation 1 h
after receiving the subcutaneous injection. Previous research has demonstrated that AMPK activity peaks between 1 and 2 h following
AICAR injection (16). The gastrocnemius muscles were rapidly dissected and frozen in liquid nitrogen with the total time elapsed for
freezing tissue being less than 60 s.
Measurement of Protein SynthesisThe fractional rate of synthesis
(Ks) was estimated from the rate of incorporation of radioactive phenylalanine into total mixed muscle protein using the specific radioactivity
of serum phenylalanine as representative of the precursor pool (17). The
actual time for incorporation of the radiolabeled phenylalanine into
protein was taken as the time elapsed from injection until freezing of
muscle in liquid nitrogen.
Analysis of Protein Kinase B (PKB)/mTOR Signaling to eIFsGastrocnemius muscles were weighed and homogenized in 7 volumes of
buffer containing 20 mM HEPES (pH 7.4), 100 mM potassium chloride,
0.2 mM EDTA, 2 mM EGTA, 50 mM sodium fluoride, 50 mM -glycerophosphate, 0.1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, 1
mM dithiothreitol (DTT), and 0.5 mM sodium vanadate. The remaining
homogenate was centrifuged at 10,000 g for 10 min at 4 C. The
resulting supernatant was combined with an equal volume of SDS
sample buffer and then subjected to protein immunoblot analysis as
described previously (18). Samples were analyzed for the phosphorylation status of 4E-BP1 on Thr37, S6K1 on Thr389, the -subunit of eIF2
(eIF2) on Ser51, PKB on Ser473, and mTOR on Ser2448 by Western blot
analysis using phosphorylation site-specific antibodies. With the exception of the anti-phospho-eIF2(Ser51) antibody, which was obtained
from BioSource International, Hopkinton, MA, the anti-phosphospecific
antibodies were obtained from Cell Signaling Technology, Beverly, MA.
Total PKB and mTOR were measured by Western blot analysis using
antibodies that recognize both the phosphorylated and unphosphorylated proteins. No change in PKB or mTOR content was observed under
any of the experimental conditions. For quantitation of the amount of
eIF4G present in the eIF4GeIF4E complex, eIF4E was immunoprecipitated from 10,000 g supernatants using a monoclonal antibody.
Samples were subjected to immunoblot analysis using a polyclonal
antibody to eIF4G to assess the association of eIF4G with eIF4E (18).
Results were normalized to the amount of eIF4E in the
immunoprecipitates.
Measurement of eIF2B ActivityThe guanine nucleotide exchange
activity of eIF2B in skeletal muscle was measured by the exchange of
[3H]GDP bound to eIF2 for nonradioactively labeled GDP as described
previously (19).
AMPK AssayIsoform-specific AMPK (1 and 2) activity was measured by homogenizing gastrocnemius muscle in ice-cold Buffer A (1:7,
w/v) containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 50 mM NaF, 5
mM sodium pyrophosphate, 1 mM EDTA, 5 mM EGTA, 1 mM DTT, 0.1
mM benzamidine, 0.1 mM phenylmethylsulfonyl fluoride, 4 mg/liter
leupeptin, 1 M microcystin, 1% Triton X-100, and 5 g/ml soybean
trypsin inhibitor. Homogenates were centrifuged at 14,000 g for 20
min at 4 C. Supernatants (150 g) were subjected to immunoprecipitation with specific antibodies to the 1 or 2 catalytic subunits of
AMPK (Upstate Biotechnology, Lake Placid, NY) and BioMag goat
anti-rabbit beads (Qiagen, Valencia, CA). Immunoprecipitates were
washed twice in Buffer A (plus 1 M NaCl and 1% Triton X-100) and twice
in Buffer A alone. Kinase reactions were performed as described previously (20).
Statistical AnalysisData are presented as means S.E. Results
were compared using a two-tailed, two-sample (equal variance) Students t test to assess differences between treatment groups. Statistical
significance was set at an level of p 0.05.
AMPK Down-regulates PKB/mTOR Signaling
23979
FIG. 2. Fractional rate of skeletal muscle protein synthesis in
response to AICAR. Protein synthesis was estimated from the rate of
incorporation of radioactive phenylalanine into total mixed muscle protein using the specific radioactivity of serum phenylalanine as representative of the precursor pool as described under Experimental Procedures (n 6 per group). *, p 0.05 versus control group.
FIG. 3. eIF4EeIF4G association and phosphorylation state of
Thr37 on 4E-BP1 and Thr389 on S6K1 following AICAR treatment
in skeletal muscle. A, eIF4E was immunoprecipitated from 10,000
g supernatants using a monoclonal antibody. Samples were subjected to
immunoblot analysis using a polyclonal antibody to eIF4G and a monoclonal antibody to eIF4E. The results are expressed as a ratio of eIF4G
to eIF4E (n 6 per group). B, phosphorylation of 4E-BP1 on Thr37 was
assessed using an anti-phospho-4E-BP1 antibody (n 10 per group). C,
phosphorylation of S6K1 on Thr389 was determined using an antiphospho-S6K1 antibody (n 10 per group). , p 0.01 versus control
group. CON, control.
To further examine potential mechanisms involved in the
depression of protein synthesis associated with 2 AMPK activation, the effect of AICAR treatment on eIF4G association
with eIF4E was examined. As shown in Fig. 3A, the amount of
eIF4G associated with eIF4E was decreased to 10% of the
control value (p 0.01) in muscle from AICAR-treated rats.
One mechanism for regulating the binding of eIF4G to eIF4E
involves phosphorylation of 4E-BP1, which releases eIF4E
from the inactive 4E-BP1eIF4E complex and allows it to bind
to eIF4G. In muscle from AICAR-treated rats, phosphorylation
of 4E-BP1 on Thr37, a priming event for phosphorylation of the
protein on additional residues that promote its dissociation
from eIF4E, was significantly reduced (p 0.01) (Fig. 3B).
Phosphorylation of Thr37 on 4E-BP1 is mediated by a protein
kinase referred to as mTOR (22). Another downstream target of
mTOR is the 70-kDa S6K1. Similar to its effect on 4E-BP1
phosphorylation, AICAR treatment reduced phosphorylation of
Thr389 on S6K1 to 5% of the value (p 0.01) observed in control
rats (Fig. 3C). Together the changes in 4E-BP1 and S6K1
phosphorylation suggest that the activity of mTOR was repressed in skeletal muscle of AICAR-treated rats.
The stimulation of the protein kinase activity of mTOR by
growth factors such as insulin or insulin-like growth factor-I is
mediated in part by phosphorylation of protein kinase B on
Ser473, which results in its activation (23). PKB subsequently
phosphorylates a residue (Ser2448) on mTOR that is present in
a domain that normally acts to repress mTOR protein kinase
activity (24). To examine whether or not activation of AMPK
might result in changes in PKB activity and thereby alter
phosphorylation of mTOR, protein immunoblot analysis was
performed using antibodies specific for mTOR phosphorylated
on Ser2448 and PKB phosphorylated on Ser473. As shown in Fig.
4, A and B, the relative phosphorylation of both mTOR on
Ser2448 and PKB on Ser473 in AICAR-treated rats was proportionately decreased to 40% of the control value (p 0.05).
DISCUSSION
AMPK is recognized as having a well established role in the
regulation of energy production and nutrient flux within skeletal muscle during periods of energetic stress. The present
study provides the first in vivo evidence that AMPK activation
directly affects translational initiation and protein synthesis in
skeletal muscle and that these responses are mediated through
the mTOR signaling pathway. The results also further establish AMPK as a unique energy sensor that not only modulates
glucose and fatty acid metabolism but also appears to regulate,
Downloaded from http://www.jbc.org/ by guest on May 1, 2016
FIG. 4. Phosphorylation state of Ser473 on PKB and Ser2448 on
mTOR in response to AICAR treatment in skeletal muscle. A,
phosphorylation of PKB on Ser473 was determined using an anti-phospho-PKB antibody (n 10 per group). B, phosphorylation of mTOR on
Ser2448 was assessed using an anti-phospho-mTOR antibody (n 6 per
group). *, p 0.05 versus control group. CON, control.
23980
AMPK Down-regulates PKB/mTOR Signaling
2
D. R. Bolster, S. J. Crozier, S. R. Kimball, and L. S. Jefferson,
unpublished observations.
Therefore, AMPK may act as a molecular signal to control
mRNA translation depending on the cellular adenine nucleotide ratios.
In conclusion, this research identifies a new cellular function
for AMPK by its ability to modulate skeletal muscle protein
synthesis and the phosphorylation state of translation initiation factors upon activation. Given the high energy consumption associated with protein synthesis in the cell, this anabolic
function may be suppressed while cellular energy is either
conserved or partitioned to maintain ATP concentrations. Furthermore, gaining a mechanistic appreciation for the regulation of skeletal muscle protein synthesis during an acute or
chronic energy stress may provide nutritional and/or therapeutic strategies for the treatment of metabolic diseases. Ultimately AMPK should be viewed with an integrated approach
by understanding its role in cellular function and how these
events can potentially influence whole body metabolism of carbohydrate, fat, and protein.
AcknowledgmentsWe thank Lynne Hugendubler, Sharon Rannels,
and Susan Nguyen for expert technical assistance and Kristen Rice for
determining the specific radioactivity of serum phenylalanine.
REFERENCES
1. Ponticos, M., Lu, Q. L., Morgan, J. E., Hardie, D. G., Partridge, T. A., and
Carling, D. (1998) EMBO J. 17, 1688 1699
2. Winder, W. W. (2001) J. Appl. Physiol. 91, 10171028
3. Hardie, D. G., Carling, D., and Carlson, M. (1998) Annu. Rev. Biochem. 67,
821 855
4. Merrill, G. F., Kurth, E. J., Hardie, D. G., and Winder, W. W. (1997) Am. J.
Physiol. 273, E1107E1112
5. Zheng, D., MacLean, P. S., Pohnert, S. C., Knight, J. B., Olson, A. L., Winder,
W. W., and Dohn, G. L. (2001) J. Appl. Physiol. 91, 10731083
6. Zhou, M., Lin, B. Z., Coughlin, S., Valega, G., and Pilch, P. F. (2000) Am. J.
Physiol. 279, E622E629
7. Bergeron, R., Ren, J. M., Cadman, K. S., Moore, I. K., Perret, P., Pypaert, M.,
Young, L. H., Semenkovich, C. F., and Shulman, G. I. (2001) Am. J. Physiol.
281, E1340 E1346
8. Corton, J. M., Gillespie, J. G., Hawley, S. A., and Hardie, D. G. (1995) Eur.
J. Biochem. 229, 558 565
9. Munday, M. R., Milic, M. R., Takhar, S., Holness, M. J., and Sugden, M. C.
(1991) Biochem. J. 280, 733737
10. Musi, N., Hayashi, T., Fujii, N., Hirshman, M. F., Witters, L. A., and Goodyear,
L. J. (2001) Am. J. Physiol. 280, E677E684
11. Vavvas, D., Apazidis, A., Saha, A. K., Gamble, J., Patel, A., Kemp, B. E.,
Witters, L. A., and Ruderman, N. B. (1997) J. Biol. Chem. 272,
1325513261
12. Chen, Z. P., McConell, G. K., Mitchell, B. J., Snow, R. J., Canny, B. J., and
Kemp, B. E. (2000) Am. J. Physiol. 279, E1202E1206
13. Hardie, D. G., and Hawley, S. A. (2001) BioEssays 23, 11121119
14. Pain, V. M. (1996) J. Biochem. 236, 747771
15. Garlick, P. J., McNurlan, M. A., and Preedy, V. R. (1980) Biochem. J. 192,
719 723
16. Holmes, B. F., Kurth-Kraczek, E. J., and Winder, W. W. (1999) J. Appl.
Physiol. 87, 1990 1995
17. Kimball, S. R., Vary, T. C., and Jefferson, L. S. (1992) Biochem. J. 286,
263268
18. Kimball, S. R., Jurasinski, C. V., Lawrence, J. C., and Jefferson, L. S. (1997)
Am. J. Physiol. 272, C754 C759
19. Kimball, S. R., and Jefferson, L. S. (1988) Biochem. Biophys. Res. Commun.
156, 706 711
20. Hardie, D. G., Salt, I. P., and Davies, S. P. (2000) in Stress Response: Methods
and Protocols (Keyse, S. M., ed) Vol. 99, pp. 6374, Humana Press Inc.,
Totowa, N. J.
21. Kimball, S. R., and Jefferson, L. S. (1994) Biochimie (Paris) 76, 729 736
22. Gingras, A.-G., Raught, B., and Sonenberg, N. (2001) Genes Dev. 15, 807 826
23. Alessi, D. R., Andjelkovic, M., Caudwell, B., Cron, P., Morrice, N., Cohen, P.,
and Hemmings, B. A. (1996) EMBO J. 15, 6541 6551
24. Sekulic, A., Hudson, C. C., Homme, J. L., Peng, Y., Otterness, D. M., Karnitz,
L. M., and Abraham, R. T. (2000) Cancer Res. 60, 3504 3513
25. Cherel, Y., Attaix, D., Rosolowska-Huszcz, D., Belkhou, R., Robin, J. P., Arnal,
M., and Le Maho, Y. (1991) Clin. Sci. (Colch.) 81, 611 619
26. Dohm, G. L., Kasperek, G. J., Tapscott, E. B., and Beecher, G. R. (1980)
Biochem. J. 188, 255262
27. Rennie, M., Edwards, R., Krywawych, S., Davies, C., Halliday, D., Warelow, J.,
and Millward, D. (1981) Clin. Sci. (Lond.) 61, 627 639
28. Wolfe, R., Goodenough, M., Wolfe, M., Royle, G., and Nadel, E. (1982) J. Appl.
Physiol. 52, 458 466
29. Tisdale, M. J. (2000) Nutrition 16, 10131014
30. Haghighat, A., Mader, S., Pause, A., and Sonenberg, N. (1995) EMBO J. 14,
57015709
31. Dennis, P. B., Jaeschke, A., Saitoh, M., Fowler, B., Kozma, S. C., and Thomas,
G. (2001) Science 294, 11021105
Downloaded from http://www.jbc.org/ by guest on May 1, 2016
in part, the anabolic functions of mRNA translation and mixed
muscle protein synthesis.
Our data reveal that 2 AMPK activation by AICAR treatment results in significant suppression of the synthesis of total
mixed muscle protein. Various metabolic states involving acute
and chronic energetic imbalances, such as fasting, exercise,
anorexia, and cachexia, have all been shown to depress protein
synthesis (2529). Identifying a molecular signal that initiates
changes in mRNA translation and protein synthesis under
these conditions has remained elusive. The concept of AMPK
acting as an energy sensor is reinforced by the present investigation by establishing increased AMPK activity as a regulator of skeletal muscle protein synthesis under conditions of an
energetic stress.
The dramatic suppression of skeletal muscle protein synthesis observed in the present study was mediated by alterations
in several key steps in translation initiation. Phosphorylation
of Thr37 on 4E-BP1 and Thr389 on S6K1 was significantly
reduced with AICAR, indicative of a decrease in global rates of
protein synthesis as well as the synthesis of specific proteins
involved with the translational apparatus (mRNAs containing
a terminal oligopyrimidine tract adjacent to the m7GTP cap, i.e.
TOP mRNA), respectively. The hypophosphorylated 4E-BP1
would be expected to sequester eIF4E and prevent its association with eIF4G (30). Indeed the profound decrease in eIF4E
associated with eIF4G observed in AICAR-treated rats corroborates this idea. Because the binding of eIF4EmRNA complex
to the ribosome requires association of eIF4E with eIF4G, the
decreased binding of eIF4G to eIF4E observed in AICARtreated rats would contribute to the depression in protein synthesis noted.
The role of eIF2 in regulating mRNA translation with AMPK
activation appears less clear. A reduction in eIF2B activity
would be suggestive of a decrease in global rates of protein
synthesis, but eIF2B activity remained unchanged with AICAR
treatment. Moreover, the small reduction in eIF2 phosphorylation is somewhat paradoxical given that such a change should
be associated with enhanced ternary complex formation, MettRNAi binding to ribosomes, and protein synthesis. The possibility exists that the diminished eIF2 phosphorylation was
part of a response that represented an attempt by the cell to
maintain a basal level of protein synthesis to prevent extensive
catabolism. Nonetheless the function of eIF2 may not be crucial
to regulating translation initiation under these conditions.
This study represents the first investigation to demonstrate
alterations in mTOR phosphorylation and accompanying
changes in 4E-BP1 and S6K1 using an in vivo model. Our
results suggest that AMPK may signal through PKB to downregulate the activity of mTOR and its downstream effectors.
The exact mechanism(s) by which AMPK modulates PKB/
mTOR phosphorylation is unknown; however, in addition to
Ser473, phosphorylation of Ser308 on PKB was also reduced2
suggesting kinases upstream of PKB (e.g. PDK1 or PDK2) may
be targets for AMPK. Moreover, the possible involvement of
changes in phosphatase activity (e.g. PP1 or PP2A) cannot be
ruled out.
A recent investigation has proposed that mTOR serves as an
energy sensor by monitoring changes in ATP concentrations
(31). However, significant decreases in ATP concentrations in
vivo are difficult to demonstrate under physiological conditions. Additionally, AMPK activation appears to be more sensitive to alterations in the ratio of AMP/ATP and phosphocreatine/creatine than to absolute changes in ATP concentrations.
AMP-activated Protein Kinase Suppresses Protein Synthesis in Rat Skeletal Muscle
through Down-regulated Mammalian Target of Rapamycin (mTOR) Signaling
Douglas R. Bolster, Stephen J. Crozier, Scot R. Kimball and Leonard S. Jefferson
J. Biol. Chem. 2002, 277:23977-23980.
doi: 10.1074/jbc.C200171200 originally published online May 7, 2002
Access the most updated version of this article at doi: 10.1074/jbc.C200171200
Alerts:
When this article is cited
When a correction for this article is posted
Click here to choose from all of JBC's e-mail alerts
Downloaded from http://www.jbc.org/ by guest on May 1, 2016
This article cites 31 references, 12 of which can be accessed free at
http://www.jbc.org/content/277/27/23977.full.html#ref-list-1
You might also like
- AMP-activated Protein Kinase Is Activated As A Consequence J. Biol. Chem.-2008-Gauthier-16514-24Document12 pagesAMP-activated Protein Kinase Is Activated As A Consequence J. Biol. Chem.-2008-Gauthier-16514-24evanconstantine77No ratings yet
- NIH Public Access: Author ManuscriptDocument34 pagesNIH Public Access: Author Manuscriptcamilamadrid01No ratings yet
- AMPK Modulates Tissue and Organismal Aging in A Non-Cell-Autonomous MannerDocument15 pagesAMPK Modulates Tissue and Organismal Aging in A Non-Cell-Autonomous MannerPilar Bravo SalasNo ratings yet
- CDD Is 2013151 ADocument10 pagesCDD Is 2013151 ADalton JLNo ratings yet
- Central Exercise Action Increases The AMPK and mTOR Response To LeptinDocument17 pagesCentral Exercise Action Increases The AMPK and mTOR Response To LeptinMario PérezNo ratings yet
- Appl1 Scaffolds Tak1-Mkk3-p38 Mapk in Adiponectin PathwayDocument9 pagesAppl1 Scaffolds Tak1-Mkk3-p38 Mapk in Adiponectin PathwaypopopioNo ratings yet
- AMPK Phosphorylation Assay KitDocument3 pagesAMPK Phosphorylation Assay KitBioAssay 2015No ratings yet
- Chen2012 Article Α-LipoicAcidRegulatesLipidMetaDocument12 pagesChen2012 Article Α-LipoicAcidRegulatesLipidMetaJoshNo ratings yet
- Glikogen 6Document12 pagesGlikogen 6DZoel FAcHMyNo ratings yet
- Metformin Induces Rab4 Through AMPK and Modulates GLUT4 Translocation in Skeletal Muscle CellsDocument8 pagesMetformin Induces Rab4 Through AMPK and Modulates GLUT4 Translocation in Skeletal Muscle CellsZulvina FaozanudinNo ratings yet
- 1 s2.0 S0753332221009720 MainDocument10 pages1 s2.0 S0753332221009720 MaingabrielaNo ratings yet
- AMPK in Skeletal Muscle Function and Metabolism 2018Document38 pagesAMPK in Skeletal Muscle Function and Metabolism 2018Rita De Cassia Marqueti DuriganNo ratings yet
- Lab Invest 2014131 ADocument12 pagesLab Invest 2014131 AGeorge Sebastian AntonyNo ratings yet
- Inhibition of Rat PC12 Cell Calpain Activity by Glutathione, Oxidized Glutathione and Nitric OxideDocument4 pagesInhibition of Rat PC12 Cell Calpain Activity by Glutathione, Oxidized Glutathione and Nitric OxidePaoloNo ratings yet
- Carling 2004Document7 pagesCarling 2004Jocilene Dantas Torres NascimentoNo ratings yet
- Ampk and The Adaptation To ExerciseDocument3 pagesAmpk and The Adaptation To ExerciseAndrés ChávezNo ratings yet
- Biochimica Et Biophysica Acta: Maayan Barnea, Liyan Haviv, Roee Gutman, Nava Chapnik, Zecharia Madar, Oren FroyDocument11 pagesBiochimica Et Biophysica Acta: Maayan Barnea, Liyan Haviv, Roee Gutman, Nava Chapnik, Zecharia Madar, Oren FroyFernando Caro MenaNo ratings yet
- Ca2calmodulin-Dependent Protein Kinase Kinase Is IDocument11 pagesCa2calmodulin-Dependent Protein Kinase Kinase Is IJoshNo ratings yet
- Ampk Hormonal 2Document11 pagesAmpk Hormonal 2miguel contrerasNo ratings yet
- Ref 17Document10 pagesRef 17Lateecka R KulkarniNo ratings yet
- Pancreatic B-Cell Response To Increased Metabolic Demand and To Pharmacologic Secretagogues Requires EPAC2ADocument12 pagesPancreatic B-Cell Response To Increased Metabolic Demand and To Pharmacologic Secretagogues Requires EPAC2AMunib Ur RehmanNo ratings yet
- 926.full 2Document3 pages926.full 2Nadilla NatashaNo ratings yet
- Lecture 8-CAMP and Cell SignallingDocument7 pagesLecture 8-CAMP and Cell Signallingjosphat nzuvaNo ratings yet
- High-Fat Diet Induces Endothelial Dysfunction Through A Down-Regulation of The Endothelial AMPK-PI3K-Akt-eNOS PathwayDocument13 pagesHigh-Fat Diet Induces Endothelial Dysfunction Through A Down-Regulation of The Endothelial AMPK-PI3K-Akt-eNOS PathwayJorge CamargoNo ratings yet
- FullDocument10 pagesFullWalida FadillahNo ratings yet
- Jurnal ResveratrolDocument10 pagesJurnal ResveratrolAsis FitrianaNo ratings yet
- 2016-Joo-AMPK Phosphorylates NRF2 Ser550Document12 pages2016-Joo-AMPK Phosphorylates NRF2 Ser550HaiNo ratings yet
- AMPK Promove Expressão Gênica de GLUT 4 em Humanos. MCGGE (2008)Document8 pagesAMPK Promove Expressão Gênica de GLUT 4 em Humanos. MCGGE (2008)Anderson Ranieri MassahudNo ratings yet
- Involvement of SIK2/TORC2 Signaling Cascade in The Regulation of Insulin-Induced PGC-1 and UCP-1 Gene Expression in Brown AdipocytesDocument10 pagesInvolvement of SIK2/TORC2 Signaling Cascade in The Regulation of Insulin-Induced PGC-1 and UCP-1 Gene Expression in Brown Adipocytesalejandra soledad alvarado neiraNo ratings yet
- MainDocument8 pagesMainAry MadinaNo ratings yet
- RNABPDocument7 pagesRNABPDr-Dalya ShakirNo ratings yet
- p38 Mitogen-Activated Protein Kinase Is The Central Regulator of Cyclic AMP-Dependent Transcription of The Brown Fat Uncoupling Protein 1 GeneDocument11 pagesp38 Mitogen-Activated Protein Kinase Is The Central Regulator of Cyclic AMP-Dependent Transcription of The Brown Fat Uncoupling Protein 1 GeneAlmir FilsNo ratings yet
- First PaperDocument8 pagesFirst PaperHanan AhmedNo ratings yet
- 2006 Gouda Et Al.Document6 pages2006 Gouda Et Al.Lalitha R GowdaNo ratings yet
- Biochemistry and Biophysics Reports: SciencedirectDocument5 pagesBiochemistry and Biophysics Reports: SciencedirectDAVID VILLANo ratings yet
- CAMPing in The Immune System - News & Announcements - Cayman ChemicalDocument3 pagesCAMPing in The Immune System - News & Announcements - Cayman ChemicalRovin RamphalNo ratings yet
- HMG-CoA Reductase Inhibitors (Statins) Activate Expression of PPARalpha - PPARgamma and ABCA1 in Cultured Gallbladder Epithelial Cells.Document8 pagesHMG-CoA Reductase Inhibitors (Statins) Activate Expression of PPARalpha - PPARgamma and ABCA1 in Cultured Gallbladder Epithelial Cells.Tiago TorresNo ratings yet
- Cold Spring Harb Symp Quant BiolDocument6 pagesCold Spring Harb Symp Quant BioleliNo ratings yet
- tmp90FE TMPDocument10 pagestmp90FE TMPFrontiersNo ratings yet
- Nihms 341890Document18 pagesNihms 341890Alex Fit FitNo ratings yet
- Junior Year Fall - Biochemistry pfk-1 Assay Formal Report Nov 30Document7 pagesJunior Year Fall - Biochemistry pfk-1 Assay Formal Report Nov 30api-651131417No ratings yet
- Froy Et Al 2011Document10 pagesFroy Et Al 2011Roee GutmanNo ratings yet
- HanooDocument8 pagesHanoomr samoNo ratings yet
- Curcumin Effect On ObesityDocument7 pagesCurcumin Effect On ObesityAldo MataNo ratings yet
- Rac1/Pi3K/Pkb-Dependent Caspase-1 Activation Release Through Hmg-Coa Reductase Inhibition Induces Il-1Document12 pagesRac1/Pi3K/Pkb-Dependent Caspase-1 Activation Release Through Hmg-Coa Reductase Inhibition Induces Il-1Josse BouwhuisNo ratings yet
- Research Proposal-Kelsi GelleDocument5 pagesResearch Proposal-Kelsi Gelleapi-619041501No ratings yet
- ANEMIA OLAHRAGA PentingDocument6 pagesANEMIA OLAHRAGA PentingfahmiNo ratings yet
- Dewei Ye, Yudong Wang, Huating Li, Weiping Jia, Kwan Man, Chung Mau Lo, Yu Wang, Karen S.L. Lam, and Aimin XuDocument13 pagesDewei Ye, Yudong Wang, Huating Li, Weiping Jia, Kwan Man, Chung Mau Lo, Yu Wang, Karen S.L. Lam, and Aimin XuzaifNo ratings yet
- Curcumin Modulates The Apolipoprotein B mRNA EditiDocument9 pagesCurcumin Modulates The Apolipoprotein B mRNA EditiLight YagamiNo ratings yet
- Autophagy & TomorigenisisDocument22 pagesAutophagy & TomorigenisisKirk CobainNo ratings yet
- Xiong2011 2Document6 pagesXiong2011 2Alejandro SalazarNo ratings yet
- The Jak/Stat Pathway: A Novel Way To Regulate PI3K ActivityDocument4 pagesThe Jak/Stat Pathway: A Novel Way To Regulate PI3K ActivityskljoleNo ratings yet
- Literature ReviewDocument15 pagesLiterature Reviewapi-619041501No ratings yet
- New Mechanisms of Metformin Action: Focusing On Mitochondria and The GutDocument10 pagesNew Mechanisms of Metformin Action: Focusing On Mitochondria and The GutZulvina FaozanudinNo ratings yet
- Biochemistry: ADP ADP Phosphorylation (Kinase) PDocument7 pagesBiochemistry: ADP ADP Phosphorylation (Kinase) PVivek VinayakumarNo ratings yet
- Diet Effects On MitochondriaDocument26 pagesDiet Effects On MitochondriabenZen100% (1)
- Regulation of Apoa Gene Expression With Acidosis: Requirement For A Transcriptional RepressorDocument15 pagesRegulation of Apoa Gene Expression With Acidosis: Requirement For A Transcriptional RepressorAbdur Rachman Ba'abdullahNo ratings yet
- Peroxisome Proliferator-Activated Receptor Gamma (Ppar C) Regulates Lactase Expression and Activity in The GutDocument11 pagesPeroxisome Proliferator-Activated Receptor Gamma (Ppar C) Regulates Lactase Expression and Activity in The GutrcastacNo ratings yet
- tmp5 TMPDocument8 pagestmp5 TMPFrontiersNo ratings yet
- Cellular Endocrinology in Health and DiseaseFrom EverandCellular Endocrinology in Health and DiseaseAlfredo Ulloa-AguirreNo ratings yet
- 2005 Return To Official Italian First Division Soccer Games Within 90 Days After Anterior Cruciate Ligament Reconstruction A Case ReportDocument15 pages2005 Return To Official Italian First Division Soccer Games Within 90 Days After Anterior Cruciate Ligament Reconstruction A Case ReportFrancisco Antonó Castro WeithNo ratings yet
- 2018 The Truth About Artificial Sweeteners - Are They Good For Diabetics...Document3 pages2018 The Truth About Artificial Sweeteners - Are They Good For Diabetics...Francisco Antonó Castro WeithNo ratings yet
- 2018 Muscle Strength Is A Poor Screening Test For Predicting Lower Extremity Injuries in Professional Male Soccer Players. A 2-Year Prospective Cohort StudyDocument11 pages2018 Muscle Strength Is A Poor Screening Test For Predicting Lower Extremity Injuries in Professional Male Soccer Players. A 2-Year Prospective Cohort StudyFrancisco Antonó Castro WeithNo ratings yet
- 2000 Metabolically Active Components of Fat Free Mass and Resting Energy Expenditure in Nonobese AdultsDocument8 pages2000 Metabolically Active Components of Fat Free Mass and Resting Energy Expenditure in Nonobese AdultsFrancisco Antonó Castro WeithNo ratings yet
- 2018 Association of Efficacy of Resistance Exercise Training With Depressive Symtoms. Meta-Analysis and Meta - Regression Analysis of Randomized Clinical TrialsDocument11 pages2018 Association of Efficacy of Resistance Exercise Training With Depressive Symtoms. Meta-Analysis and Meta - Regression Analysis of Randomized Clinical TrialsFrancisco Antonó Castro WeithNo ratings yet
- 2018 Physical Therapists Forward Deployed On Aircraft Carriers A Retrospective Look at A Decade of ServiceDocument6 pages2018 Physical Therapists Forward Deployed On Aircraft Carriers A Retrospective Look at A Decade of ServiceFrancisco Antonó Castro WeithNo ratings yet
- 2018 A Systematic Review of The Relationship Between Physical Activity and HappinessDocument18 pages2018 A Systematic Review of The Relationship Between Physical Activity and HappinessFrancisco Antonó Castro Weith100% (1)
- 2004 Ability of A New Hop Test To Determine Functional Deficits After Anterior Cruciate Ligament ReconstructionDocument7 pages2004 Ability of A New Hop Test To Determine Functional Deficits After Anterior Cruciate Ligament ReconstructionFrancisco Antonó Castro WeithNo ratings yet
- 2018 The Truth About Artificial Sweeteners - Are They Good For Diabetics...Document3 pages2018 The Truth About Artificial Sweeteners - Are They Good For Diabetics...Francisco Antonó Castro WeithNo ratings yet
- 2018 Football Recovery Strategies. Practical Aspects of Blending Science and RealityDocument8 pages2018 Football Recovery Strategies. Practical Aspects of Blending Science and RealityFrancisco Antonó Castro Weith100% (1)
- 2018 Cardiovascular and Metabolic Heterogeneity of Obesity. Clinical Challenges and Implications For ManagementDocument25 pages2018 Cardiovascular and Metabolic Heterogeneity of Obesity. Clinical Challenges and Implications For ManagementFrancisco Antonó Castro WeithNo ratings yet
- 2018 Evaluation of Spine MRIs in Athletes Participating in The Rio de Janeiro 2016 Summer Olympic GamesDocument8 pages2018 Evaluation of Spine MRIs in Athletes Participating in The Rio de Janeiro 2016 Summer Olympic GamesFrancisco Antonó Castro WeithNo ratings yet
- 2018 Match Running Performance in Professional Soccer Players Effect of Match Status and Goal DifferenceDocument3 pages2018 Match Running Performance in Professional Soccer Players Effect of Match Status and Goal DifferenceFrancisco Antonó Castro WeithNo ratings yet
- 2018 Muscle Strength Is A Poor Screening Test For Predicting Lower Extremity Injuries in Professional Male Soccer Players. A 2-Year Prospective Cohort StudyDocument11 pages2018 Muscle Strength Is A Poor Screening Test For Predicting Lower Extremity Injuries in Professional Male Soccer Players. A 2-Year Prospective Cohort StudyFrancisco Antonó Castro WeithNo ratings yet
- 2012 Recovery After High-Intensity Intermittent Exercise in Elite Soccer Players Using VEINOPLUS Sport Technology For Blood-Flow StimulationDocument9 pages2012 Recovery After High-Intensity Intermittent Exercise in Elite Soccer Players Using VEINOPLUS Sport Technology For Blood-Flow StimulationFrancisco Antonó Castro WeithNo ratings yet
- 2003 Hindlimb Unloading Alters Ligament HealingDocument11 pages2003 Hindlimb Unloading Alters Ligament HealingFrancisco Antonó Castro WeithNo ratings yet
- 2017 Genes To Predict VO2max Trainability A Systematic ReviewDocument30 pages2017 Genes To Predict VO2max Trainability A Systematic ReviewFrancisco Antonó Castro WeithNo ratings yet
- 2016 Genes and Athletic Performance An UpdateDocument15 pages2016 Genes and Athletic Performance An UpdateFrancisco Antonó Castro WeithNo ratings yet
- 2014 Kinematic Analysis of The Instep Kick in Youth Soccer PlayersDocument10 pages2014 Kinematic Analysis of The Instep Kick in Youth Soccer PlayersFrancisco Antonó Castro WeithNo ratings yet
- 1996 Effects of Moderate-Intensity Endurance and High-Intensity Intermittent Training On Anaerobic Capacity and VO (2max)Document12 pages1996 Effects of Moderate-Intensity Endurance and High-Intensity Intermittent Training On Anaerobic Capacity and VO (2max)Francisco Antonó Castro WeithNo ratings yet
- 2016 Effects of Low Versus High-Load Resistance Training On Muscle Strength and Hypertrophy in Well-Trained MenDocument32 pages2016 Effects of Low Versus High-Load Resistance Training On Muscle Strength and Hypertrophy in Well-Trained MenFrancisco Antonó Castro WeithNo ratings yet
- Human Movement Science: Full Length ArticleDocument10 pagesHuman Movement Science: Full Length ArticleFrancisco Antonó Castro WeithNo ratings yet
- 2016 Effects of Mental Imagery On Muscular Strength in Healthy and Patient Participant A Systematic ReviewDocument17 pages2016 Effects of Mental Imagery On Muscular Strength in Healthy and Patient Participant A Systematic ReviewFrancisco Antonó Castro WeithNo ratings yet
- 2016 Visceral Adiposity and Metabolic Syndrome After Very High-Fat and Low-Fat Isocaloric Diets A Randomized Controlled TrialDocument15 pages2016 Visceral Adiposity and Metabolic Syndrome After Very High-Fat and Low-Fat Isocaloric Diets A Randomized Controlled TrialFrancisco Antonó Castro WeithNo ratings yet
- 2017 Association of Weekend Warrior and Other Leisure Time Physical Activity Patterns With Risks For All-Cause, Cardiovascular Disease, and Cancer MortalityDocument8 pages2017 Association of Weekend Warrior and Other Leisure Time Physical Activity Patterns With Risks For All-Cause, Cardiovascular Disease, and Cancer MortalityFrancisco Antonó Castro WeithNo ratings yet
- 2017 Collateral Fattening When A Deficit in Lean Body Mass Drives OvereatingDocument3 pages2017 Collateral Fattening When A Deficit in Lean Body Mass Drives OvereatingFrancisco Antonó Castro WeithNo ratings yet
- 2016 Enhanced Muscle Insulin Sensitivity After Contraction-Exercise Is Mediated by AMPKDocument37 pages2016 Enhanced Muscle Insulin Sensitivity After Contraction-Exercise Is Mediated by AMPKFrancisco Antonó Castro WeithNo ratings yet
- 2016 Inverse Relationship Between Sleep Duration and Cardio-Ankle Vascular Index in ChildrenDocument8 pages2016 Inverse Relationship Between Sleep Duration and Cardio-Ankle Vascular Index in ChildrenFrancisco Antonó Castro WeithNo ratings yet
- 2009 Muscle Fatigue in Males and Females During Multiple-Sprint ExerciseDocument22 pages2009 Muscle Fatigue in Males and Females During Multiple-Sprint ExerciseFrancisco Antonó Castro WeithNo ratings yet
- 2016 Developmental Timing and Critical Windows For The Treatment of Psychiatric DisordersDocument10 pages2016 Developmental Timing and Critical Windows For The Treatment of Psychiatric DisordersFrancisco Antonó Castro WeithNo ratings yet
- Nina Fay Calhoun Award - Intl RelationsDocument5 pagesNina Fay Calhoun Award - Intl RelationsAltrusa International of Montrose CONo ratings yet
- The Good-Enough Sex Model For Couple Sexual SatisfactionDocument13 pagesThe Good-Enough Sex Model For Couple Sexual SatisfactionwernikNo ratings yet
- Grade 8 - P.E PPT (Week 1)Document30 pagesGrade 8 - P.E PPT (Week 1)Dave Sedigo100% (1)
- Final Sheet MotalityDocument69 pagesFinal Sheet MotalityAshima GabgotraNo ratings yet
- Mental Health Awareness SpeechDocument2 pagesMental Health Awareness SpeechThea Angela Longino95% (20)
- 2022 HRM Systems Diagnostic ChecklistsDocument5 pages2022 HRM Systems Diagnostic ChecklistsSacred EsportsNo ratings yet
- Part-IDocument507 pagesPart-INaan SivananthamNo ratings yet
- The Effect of Narcotic DrugDocument4 pagesThe Effect of Narcotic DrugFasra ChiongNo ratings yet
- Nurse - Resignation LetterDocument1 pageNurse - Resignation LetterphoenixdashNo ratings yet
- Receiving and Storage PDFDocument12 pagesReceiving and Storage PDFshyamkattiNo ratings yet
- Umbar - Performance Task 7Document3 pagesUmbar - Performance Task 7Bella CiaoNo ratings yet
- Job Safety Analysis Worksheet: JSA JSA Participants PPE Required Tools And/or EquipmentDocument5 pagesJob Safety Analysis Worksheet: JSA JSA Participants PPE Required Tools And/or EquipmentVigieNo ratings yet
- AishwaryaDocument52 pagesAishwaryamohitNo ratings yet
- FA Form No2-Visa Application FormDocument1 pageFA Form No2-Visa Application FormacademydonutNo ratings yet
- AICTE CorporateBestPracticesDocument13 pagesAICTE CorporateBestPracticesramar MNo ratings yet
- Wellness at SeaDocument9 pagesWellness at SeaRam Niwas ChauhanNo ratings yet
- A Solution-Focused Approach To Rational-Emotive Behavior Therapy - Toward A Theoretical IntegrationDocument22 pagesA Solution-Focused Approach To Rational-Emotive Behavior Therapy - Toward A Theoretical Integrationsolutions4familyNo ratings yet
- Gastritis - Etiology and Diagnosis - UpToDateDocument10 pagesGastritis - Etiology and Diagnosis - UpToDateLizbeth Navarrete SierraNo ratings yet
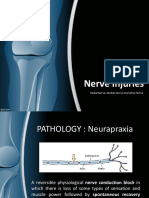
- Nerve Injuries: Radial Nerve, Median Nerve and Ulnar NerveDocument26 pagesNerve Injuries: Radial Nerve, Median Nerve and Ulnar NerveaisiyaahNo ratings yet
- Effective Leadership Towards The Star Rating Evaluation of Malaysian Seni Gayung Fatani Malaysia Organization PSGFMDocument10 pagesEffective Leadership Towards The Star Rating Evaluation of Malaysian Seni Gayung Fatani Malaysia Organization PSGFMabishekj274No ratings yet
- Tumors of Head and Neck RegionDocument94 pagesTumors of Head and Neck Regionpoornima vNo ratings yet
- Philippines AFHS - Standards and Implementation GuideDocument37 pagesPhilippines AFHS - Standards and Implementation GuideShardin Labawan-Juen,RNNo ratings yet
- EDC Annual ReportDocument433 pagesEDC Annual ReportAngela CanaresNo ratings yet
- World AIDS Day - December 1, 2019 Status of HIV Case-Based Surveillance Implementation - 39 U.S. PEPFAR-Supported Countries, May-July 2019Document16 pagesWorld AIDS Day - December 1, 2019 Status of HIV Case-Based Surveillance Implementation - 39 U.S. PEPFAR-Supported Countries, May-July 2019worksheetbookNo ratings yet
- Abc Ven 2020Document81 pagesAbc Ven 2020CorneLia JacintaNo ratings yet
- Lesson 4Document4 pagesLesson 4api-316910625No ratings yet
- YDA Nepal - MembersDocument13 pagesYDA Nepal - MembersSulochan LohaniNo ratings yet
- Monitoring The Performance of DamDocument137 pagesMonitoring The Performance of Damputih_138242459100% (2)
- Galay1 1 1 1Document2 pagesGalay1 1 1 1Glynne AlmadenNo ratings yet
- SUPW SrimukhDocument15 pagesSUPW SrimukhsrimukhsaiNo ratings yet