Professional Documents
Culture Documents
Lapkas Anak SN
Uploaded by
ŘŷØoo TЯyCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Lapkas Anak SN
Uploaded by
ŘŷØoo TЯyCopyright:
Available Formats
1
CHAPTER I
INTRODUCTION
Nephrotic syndrome is a group of symptoms characterized by proteinuria,
hypoproteinemia, edema, and hyperlipidemia. It may occur as a result of any form
of glomerular disease and may rarely be associated with a variety of extrarenal
conditions.1 The term nephrotic syndrome was firstly used by Calvin and
Goldberg and this term is used to exchange the old term which shows the clinical
condition and laboratory without showing the underlying disease.2
Incidence of nephrotic syndrome in United State and England are 2-4 new
cases over 100.000 children/year, while in the developing country the incidence of
nephrotic syndrome is higher. In Indonesia, it is reported that there are 6 cases
over 100.000 children/year.3
Nephrotic syndrome is usually caused by glomerulus defect which can be
classified into primary and secondary form. The primary disorder is an increase in
glomerular permeability to proteins, most likely as a result of the loss of the
glomerular basement membrane sialoproteins, which leads to a loss of the normal
negative charge. Massive proteinuria results and leads to a decline in serum
proteins, especially albumin.4
Minimal change nephrotic syndrome (MCNS) is the most common
histologic form of nephrotic syndrome, seen in 70% to 80% of cases. Males are
affected more frequently than females by a 2 : 1 ratio. Most children younger than
7 years old with nephrotic syndrome have MCNS. Children 7 to 16 years old with
nephrotic syndrome have a 50% chance of having MCNS.5
Corticosteroid is the first choice for nephrotic syndromes medication,
unless there is a contraindication with the patient. Prednison or prednisolon can be
given to treat nephrotic syndrome. After 2 weeks of steroid therapy, usually the
remission occurs in 80% cases, and it can reach 94% of remission after 4 weeks of
steroid therapy. If there is no remission after 4 weeks initial full dose of steroid
medication, then the patient can be stated as a steroid resistant.5
Most children with nephrotic syndrome eventually go into remission.
Nearly 80% of children with MCNS experience a relapse of the proteinuria at
some point, defined as heavy proteinuria that persists for 3 to 5 consecutive days.
Transient (1 to 2 days) proteinuria may occur with an intercurrent infection in
children with MCNS and is not considered a relapse. Patients with MCNS who
respond to steroid therapy have little risk of chronic renal failure.1
CHAPTER II
LITERATURE REVIEW
2.1
Definition
Nephrotic syndrome is a clinical syndrome with symptoms as following5 :
a. Massive proteinuria (40 mg/m2 / hour or ratio protein/creatinin > 2 mg or dipstick
+2)
b. Hypoalbuminemia 2,5 g/dl
c. Edema
d. Can be followed by hypercholesterolemia
There are some definiton for the type of nephrotic syndrom5 :
a.
Remission
: proteinuria negative or trace (proteinuria < 4 mg/m 2/hour) in 3
following days during a week
b.
Relapse
: proteinuria 2+ (proteinuria 40mg/m2/hour) in 3 following
days during a week
c.
Seldom Relapse : Relapse occurs less than 2 times in 6th first month after the
initial response or less than 4 times per year.
d.
Frequent Relapse : Relapse occurs 2 times in 6th first month after the initial
response or 4 times during 1 year periode.
e.
Steroid Dependent: Relapse occurs while the dose of steroid decreasing or during
14 days after the medication has stopped, and it occurs 2 times.
f.
Steroid Resistant : There is no remission with full dose prednison medication ( 2
mg/kgBB/days) during 4 weeks.
2.2
Epidemiology
In the United States, incidence of nephrotic syndrome is
2-7 cases per
100.000 children per year and cumulative prevalence of 16 per 100.000 children.
Primary nephrotic syndrome is about 90% found in children. The prevalence of
primary nephrotic syndrome in Indonesia is about 6 per 100.000 in children below 14
years old. 3
Nephrotic syndrome is more common in boys than girls in younger age
groups, but once in adolescence, there is no significant difference between genders.
This syndrome is commonly seen at ages 3 to 5, and the incidence of this cases
increase and more severely seen in African American and Hispanic populations.3
Age, race, and geography affect the incidence of nephrotic syndrome. Certain
HLA types (HLA-DR7, HLA-B8, and HLA-B12) are associated with an increased
incidence of nephrotic syndrome.1
2.3
Etiology
Minimal change nephrotic syndrome (MCNS) is the most common
histologic form of nephrotic syndrome, seen in 70% to 80% of cases. Males are
affected more frequently than females by a 2 : 1 ratio. Most children younger than 7
years old with nephrotic syndrome have MCNS. Children 7 to 16 years old with
nephrotic syndrome have a 50% chance of having MCNS. A trial of steroid therapy is
indicated before biopsy in these older children if the presentation is typical.1
Focal segmental glomerulosclerosis (FSGS) is usually identical to that of
MCNS. FSGS may progress from MCNS or present as a separate entity. A circulating
factor that increases glomerular permeability to albumin is found in some patients
with FSGS. FSGS accounts for approximately 10% of children with nephrotic
syndrome.1
Membranous nephropathy is infrequent in childhood. Approximately 1% of
children with nephrotic syndrome have this lesion on a kidney biopsy specimen. It is
seen most commonly in adolescents and children with systemic infections, such as
hepatitis B, syphilis, malaria, and toxoplasmosis, or receiving drug therapy (gold salts,
penicillamine). Hematuria is common.1
Congenital nephrotic syndrome is defined as clinical nephrotic syndrome
that presents during the first 6 months of life. There are two common types. The
Finnish type is an autosomal recessive disorder most common in persons of
Scandinavian descent and is due to a mutation in a component protein in the
glomerulus filtration slit. The second type is a heterogeneous group of abnormalities
including diffuse mesangial sclerosis and conditions associated with drugs or
infections. Prenatal diagnosis is suspected if there are elevated levels of maternal
alpha-fetoprotein.1
Secondary forms of nephrotic syndrome may be due to systemic lupus
erythematosus and infections (syphilis, hepatitis B).4
2.4
Risk Factor
a.
Infection
Infection is an important cause of relapse in nephrotic syndrome. An upper
respiratory tract infection (URTI) or a febrile episode often precipitates a relapse and
occasionally there is no obvious cause. Asymptomatic UTI might also be an important
and under diagnosed cause of relapse.6
b.
Protein Serum
Young age and low level of serum protein at onset are independent risk for
relapse. The research of MN Sarker et.al in 2005 shows that the low serum albumin
and total protein level at the time of initial attack were significantly associated with
frequent relapse of nephrotic syndrome.6
c.
Social & Economy
In our country, poverty, inadequate health care facility, less organized referral
system, and lack of adequate knowledge about disease course among parents are great
problem in early detection and treatment of relapse cases.6
2.5
Pathophysiology
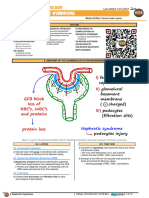
Normally, the glomerular filtration barrier is composed of 3 layers, listed
from capillary side to bowmans space side:
Fenestrated endothelium
Glomerular basement membrane : Negatively charged to prevent the passage
of large anionic molecules (such as albumin)
Visceral glomerular epithelium, also known as podocytes
- Podocytes contain foot processes, which create a barrier
- Small pores between adjacent foot processes are bridged by slit
diaphragms
- Podocytes affect the structure and function of both the glomerular
basement membrane and the endothelial cells
Size discrimination is accomplished by the pores in the glomerular basement
membrane and podocytes which have a radius of approximately 40 to 45
amperes.4
In nephrotic syndrome, the normal glomerular filtration process in interrupted,
resulting in protein passing through the filtration barrier and severe-range proteinuria.
Commonly a defect is in the podocytes and/or glomerular basement membrane.
Recent experiments have implicated T-Cells in the damage to podocytes leading to 2
common types of nephrotic syndrome (minimal change disease and focal-segmental
glomerulosclerosis). Exact pathology varies depending on the specific type of
nephritic syndrome.1
Several mechanisms result in proteinuria. Impaired reabsorption of proteins by
the proximal tubule, as seen in Fanconi syndrome and drug or heavy metal exposure,
may cause significant proteinuria. Factors that increase glomerular permeability also
result
in
proteinuria.
These
factors
include
physical
damage,
abnormal
hemodynamics, and hormone-mediated changes.1
The primary disorder is an increase in glomerular permeability to proteins,
most likely as a result of the loss of the glomerular basement membrane sialoproteins,
which leads to a loss of the normal negative charge. Massive proteinuria results and
leads to a decline in serum proteins, especially albumin. Plasma oncotic pressure is
diminished, resulting in a shift of fluid from the vascular to the interstitial
compartment and a contraction in plasma volume.1
Renal blood flow and GFR are not usually diminished, and in some instances
GFR may be above normal. Edema formation is enhanced by a reduction in effective
blood volume and by an increase in tubular sodium chloride reabsorption secondary to
activation of the renin-angiotensin-aldosterone system.1
Most serum lipids (including cholesterol and triglycerides) and levels of
lipoprotein are elevated because hypoproteinemia stimulates hepatic lipoprotein
synthesis, while lipid metabolism is diminished.1
2.6
Clinical Presentation
Characteristic findings:4
Proteinuria
Hypoalbuminemia
Generalized edema
Due to a decrease in plasma oncotic pressure which follows massive albumin
urinary losses. Begins in areas with low resistance, which can be seen in
minimal change diseases characteristic eyelid swelling, or puffy eyes. It can
also lead to scrotal or vulvar edema
Hyperlipidemia
Likely due to increased hepatic production of very low-density lipoprotein
(VLDL), intermediate-density lipoprotein (IDL), and low-density lipoprotein
(LDL) in response to hypoproteinemia
2.7
Diagnosis
Diagnostic criteria (must see both)
Serum albumin below 3 g/dL
Urine protein excretion greater than 50 mg/kg per day or greater than 3.5g of
protein in a 24-hr urine sample
In the absence of identifiable systemic disease, the vast majority of patients that meet
diagnostic criteria for nephrotic syndrome have minimal change disease and will be
treated accordingly.5 Other diagnostic tests, mostly aimed at identifying pathologic
processes other than minimal change disease, include:5
Urinalysis
Hematuria can occasionally be seen in FSGS but is usually a sign of nephritic
syndrome
Protein to creatinine ration from first void of morning
UPr/Cr greater than 3.0 is consistent with nephrotic syndrome
Serum studies including electrolytes, creatinine, BUN, lipid panel, albumin,
and complement levels. Also, ANA for patients over ten years old, and
hepatitis b/c and HIV testing.
Renal biopsy if strong suspicion of pathology other than minimal change
disease
When to biopsy ?
Patients that meet all of the following criteria can be treated empirically
without renal biopsy (other patients could benefit from biopsy):
- Between ages of 1 and 10
- None of the following present: hypertension, gross hematuria, elevated
creatinine
- Normal complement levels
2.8
Differential Diagnosis
a.
Transient proteinuria
Seen after vigorous exercise and occasionally in febrile or dehydrated
children. The proteinuria usually is mild (protein-to-creatinine ratio <1) and does not
indicate renal disease.1
b.
Postural (orthostatic) proteinuria
A benign condition defined by normal protein excretion while patients are
recumbent, but significant, although moderate, proteinuria when they are upright.
"Persistent" proteinuria associated with renal disease has a positional increase during
activity, but also is present when recumbent. If proteinuria is persistent, renal disease
should be considered.1
c.
Glomerular proteinuria
It is classified by its degree. Intermittent (mild) proteinuria (<0.5 g/m 2/day) is
seen in pyelonephritis, renal cystic diseases, obstructive uropathies, and mild
glomerulonephritis. Moderate proteinuria (0.5 to 1 g/m 2/day) is seen in acute poststreptococcal
glomerulonephritis,
mild
Henoch-Schnlein
nephritis,
severe
pyelonephritis, chronic glomerulonephritis, and hemolytic uremic syndrome (HUS).
Severe proteinuria (>1 g/m2/day) is characteristically associated with nephrotic
syndrome.1
CHAPTER 3
MEDICAL RECORD
3.1. Objective
The aim of this paper is to report a case of Nephrotic Syndrome of a 1 year and 10
months old girl that was admitted at Pediatric Department of Haji Adam Malik
General Hospital.
3.2. Case
Name : ADH
Age : 1 year 10 months
Sex : Girl
Date of Admission : De, 22nd 2015
Chief of Complaint : Swelling all over the body
History :
This condition has already occured since the last 3 months ago and got worse
in a week before the day of admission. At first, the swelling occurs at the face of the
patient, then to the hands and all over the body. Cough withe phlegm is positive since
2 days before the day of admission. Shortness of breath was positive since the belly
swelled. The shortness of breath is not related to the weather of activities, orthopnea is
denied. Fever was positive since a day before the day of admission. The fever was not
so high and it reduced after consuming paracetamol.
Previous Illness :
This patient has already been treated by pediatrician in Madina Hospital, and
she was referred from that hospital with the nephrotic syndrome as the diagnosis.
History of Medication : the use of prednisone for 3 weeks
History of pregnancy and birth :
The gestation age was 37 weeks. OS born at term spontaneously and helped by
midwives with 3000 grams birth weight buth the mother did not remember the birth
body length. After born, baby cried immediately. Blue or cyanotic history not found,
yellow history not found. OS mother was pregnant when she was 31 years old.
Pregnancy examinations done regularly to the midwife. History of smoking, drinking
alcohol, drugs and herbs was not found.
Feeding History
From birth to 6 months : Breast milk
From 6 months to 1 year 2 months : breast milk with additional food
From 1 year 2 months until now : Family food
History of Growth and Development
The patients mother reported that ADH grew normally. ADH had developed
crawling, sitting, walking and talking on time.
Physical Examination
General status
Body Weight : 10,1 Kg
Body Length : 77 Cm
BW/age : -2 < Z score < 0 (normal)
BL/age: -3 < Z score < -2 (short)
10
BW/BL : 0 < Z score < 1 (well nourished)
Present Status
Conciousness : GCS 15 (E4V5M6)
Blood Pressure : 110/60 mmHg
Heart Rate : 136 t/m
Respiratory Rate : 38 t/m
Body Temperature : 37,8oC
Anemic (-); Icteric (-); Cyanosis (-); Edema (+); Jaundice (-); Dyspnea (+)
Local Status
Head : Eyes : RC +/+ pupil isochor; Anemic inferior papebra conjunctiva (-/-); Icteric
sclera (-/-); Lightreflex (+/+). Edema palpebra superior (+/+) Face edema (+);
Ear/nose/mouth : Normal.
Neck : lymph node enlargement (-); Jugula vein pressure (R-2cmH2O)
Thorax : Symmetric fusiformis; Chest retrction (-); HR (136 t/m); Murmur (-); RR (38
t/m); Breathing Sound (vesicular) with low vesicular in the lower zone of right lung.
Abdomen : distention (+), peristaltic (+) normal, undulation (+), Liver and Spleen
(hard to measure); Waist Circumference (Sit) = 41 cm, Waist Circumference (Supine)
= 38 cm.
Extremities :Pulse (138 t/m); regular; adequate pressure and volume; warm; CRT<3 s;
Pitting edema (+).
Laboratory Result : 21st September 2015
Parameters
Hemoglobin
Eritrochyte
Leukocyte
Thrombocyte
Hematocrite
MCV
MCH
MCHC
RDW
Diftel
Value
Complete Blood Count
11.80 g/dl
4.27 x 106/mm3
18.80 x 103/mm3
615 x 103/mm3
35.80 %
83.80 fl
27.60 pg
33.00 g %
16.20%
0.400/1.20/42.80/36.60/19.
Normal Value
00
2-8
11.3-14.1 g/dl
4.40-4.48 x 106/mm3
6.0-17.5 x 103/mm3
217-497 x 103/mm3
37-41 %
81-95 fl
25-29 pg
29-31 g %
11.6-14.8%
0-1/ 1-6/ 37-80/ 20-40/
11
pH
pCO2
pO2
Bicarbonat (HCO3)
Total CO2
Base Excess
O2 Saturation
Albumin
Random Blood Glucose
Ureum
Creatinin
Calcium (Ca)
Natrium (Na)
Potassium (K)
Chloride (Cl)
Blood Gas Analyze
7.420
35 mmHg
104 mmHg
22.7 mmol/L
23.8 mmol/L
-1.4 mmol/L
98 %
Liver Function
1.5 g/dl
Carbohydrate Metabolism
82.10 mg/dl
Renal Function
10.70 mg/dl
0.27 mg/dl
Electrolyte
7.6 mg/dl
134 mEq/L
4.7 mEq/L
105 mEq/L
Urine Dipstick
Proteinuria
Differential Diagnosis:
- Nephrotic Syndrome
- Acute Glomerulonephritis post Strepococcal infection
- SLE
- Heart failure
Working Diagnosis:
- Nephrotic Syndrome
Management:
- Inj Furosemide 10 mg/12 hours
- Syr Paracetamol 3 x 1 cth s.prn
- Diet MB 1000 Kkal + 20 gr protein
Diagnostic Planning:
- Hipoalbuminemia Correction
7.35-7.45
38-42 mmHg
85-100 mmHg
22-26 mmol
19-25
(-2) (+2)
96-100
3.8-5.4 g/dl
< 200 mg/dl
< 50 mg/dl
0.24-0.41 mg/dl
8.4 10.4 mg/dl
135 155 mEq/L
3.6 5.5 mEq/L
96-106
+3
12
- Lipid profile check, urinalisis
- Fluid Balance / 6 hours
- Urine Dipstick / 6 hours
REFERENCES
1. Kliegman R.M, Marcdante K.J, Jenson H.B, Behrman R.E. Nelson Essentials
of Pediatrics. Elsevier. 2007
2. Alatas H, Tambunan T, Trihono PP, Pardede SO : Sindrom Nefrotik, Buku Ajar
Nefrologi Anak. Edisi 2. Balai Penerbit FKUI, Jakarta. 2004.
3. Gordillo R. The Nephrotic Syndrome. Pediatrics in Review / American
Academy of Pediatrics. 2009
4. Lennon, R. Nephrotic Syndrome in Children. Paediatrics and Child Health.
2010
5. IDAI. Konsensus Tata Laksana Sindrom Nefrotik Idiopatik Pada Anak. Unit
13
Kerja Koordinasi Nefrologi IDAI. 2010.
6. Sarker, et al. Risk Factor for Relapse in Childhood Nephrotic Syndrome A
Hospital Based Retrospective Study. 2012
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (120)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Caprini DVT Risk AssessmentDocument2 pagesCaprini DVT Risk AssessmentAnonymous PjQxbsJQaNo ratings yet
- Review Article - Periodontitis With Diabetes Mellitus PDFDocument5 pagesReview Article - Periodontitis With Diabetes Mellitus PDFŘŷØoo TЯyNo ratings yet
- IPSGDocument16 pagesIPSGNyimas Milka Ayu NabilaNo ratings yet
- MELD Vs CPTDocument6 pagesMELD Vs CPTŘŷØoo TЯyNo ratings yet
- Review Article - Periodontitis With Diabetes Mellitus PDFDocument5 pagesReview Article - Periodontitis With Diabetes Mellitus PDFŘŷØoo TЯyNo ratings yet
- Review Article - Periodontitis With Diabetes MellitusDocument10 pagesReview Article - Periodontitis With Diabetes MellitusŘŷØoo TЯyNo ratings yet
- Vitrectomy Machine:: Nadia Artha DewiDocument21 pagesVitrectomy Machine:: Nadia Artha DewiŘŷØoo TЯyNo ratings yet
- Child Pugh ScoreDocument1 pageChild Pugh ScoreRyoFernandoNo ratings yet
- CcpcardiogenicDocument51 pagesCcpcardiogenicŘŷØoo TЯyNo ratings yet
- Pass Ms 2010Document1 pagePass Ms 2010ŘŷØoo TЯyNo ratings yet
- HP Mar00 HypocalDocument3 pagesHP Mar00 HypocalDimas Dwi HarryadiNo ratings yet
- Article34015028 PDFDocument15 pagesArticle34015028 PDFJustine May GervacioNo ratings yet
- CcpcardiogenicDocument51 pagesCcpcardiogenicŘŷØoo TЯyNo ratings yet
- ATLS Programme Draft 3Document2 pagesATLS Programme Draft 3Fernando GeldresNo ratings yet
- Drug Induced Hepatitis Tuberculosis Treatment PDFDocument6 pagesDrug Induced Hepatitis Tuberculosis Treatment PDFŘŷØoo TЯyNo ratings yet
- DiliDocument18 pagesDiliKharisma Rizqiah WahyuniNo ratings yet
- 2 PDFDocument13 pages2 PDFKarina Mega WNo ratings yet
- Jurnal PH PDFDocument5 pagesJurnal PH PDFŘŷØoo TЯyNo ratings yet
- Higher Algebra - Hall & KnightDocument593 pagesHigher Algebra - Hall & KnightRam Gollamudi100% (2)
- Glaucoma 4Document19 pagesGlaucoma 4ŘŷØoo TЯyNo ratings yet
- WR2 PDFDocument9 pagesWR2 PDFJoni MokodoNo ratings yet
- 2 PDFDocument13 pages2 PDFKarina Mega WNo ratings yet
- Poster Public HealthDocument2 pagesPoster Public HealthŘŷØoo TЯyNo ratings yet
- (Ancylostoma Braziliense) (Ancylostoma Caninum) (Ancylostoma Duodenale) (Necator Americanus)Document8 pages(Ancylostoma Braziliense) (Ancylostoma Caninum) (Ancylostoma Duodenale) (Necator Americanus)ŘŷØoo TЯyNo ratings yet
- Pathogens 01 00001Document2 pagesPathogens 01 00001ŘŷØoo TЯyNo ratings yet
- DyspepsiaDocument25 pagesDyspepsiaCynthia StefanusNo ratings yet
- Nephrotic SyndromeDocument23 pagesNephrotic SyndromeDivina Francia JovenNo ratings yet
- Glomerulopathy: Acute Nephritic SyndromeDocument109 pagesGlomerulopathy: Acute Nephritic SyndromekamalNo ratings yet
- Enfermedad GlomerularDocument23 pagesEnfermedad GlomerularAlejandro beuses morrNo ratings yet
- Glomerulonephritis EngDocument43 pagesGlomerulonephritis EngNosirova ManijaNo ratings yet
- Renal Notes Step 2ckDocument34 pagesRenal Notes Step 2cksamreen100% (1)
- Nephrotic SyndromeDocument13 pagesNephrotic Syndromeadi tiarman100% (1)
- Nephrotic Syndrome Among Children in Kano: A Clinicopathological StudyDocument6 pagesNephrotic Syndrome Among Children in Kano: A Clinicopathological StudyBANGDE TVNo ratings yet
- Renal Toxicity of Lithium - UpToDateDocument19 pagesRenal Toxicity of Lithium - UpToDateXochitl7No ratings yet
- KDIGO GD Guideline Key Takeaways For Clinicians FSGSDocument1 pageKDIGO GD Guideline Key Takeaways For Clinicians FSGSadamu mohammadNo ratings yet
- The Columbia Renal Biopsy Course: JULY 12 - JULY 14, 2023Document8 pagesThe Columbia Renal Biopsy Course: JULY 12 - JULY 14, 2023Freddy Shanner Chávez VásquezNo ratings yet
- Nephrotic Syndrome Adults 508Document6 pagesNephrotic Syndrome Adults 508yuliaevitasariNo ratings yet
- Conditions Associated With ProteinuriaDocument7 pagesConditions Associated With ProteinuriaMika SarenoNo ratings yet
- Onconephrology: Update in Anticancer Drug-Related NephrotoxicityDocument13 pagesOnconephrology: Update in Anticancer Drug-Related NephrotoxicityFreddy Shanner Chávez VásquezNo ratings yet
- Renal Pathology: Kidney and The Urinary Collecting SystemDocument37 pagesRenal Pathology: Kidney and The Urinary Collecting Systemapplesncore100% (1)
- Chapter 21 - Urologic Aspects of Pediatric Nephrology - Campbell - Walsh-Wein UROLOGY 12thDocument19 pagesChapter 21 - Urologic Aspects of Pediatric Nephrology - Campbell - Walsh-Wein UROLOGY 12thkrisnaNo ratings yet
- Nephrotic Syndrome in Children: by Prof Grace W. Irimu (Mmed, PHD)Document46 pagesNephrotic Syndrome in Children: by Prof Grace W. Irimu (Mmed, PHD)okwadha simionNo ratings yet
- PH Protocol For Nephrotic SyndromeDocument4 pagesPH Protocol For Nephrotic Syndromeprachi100% (1)
- Nephrotic Syndrom WardDocument49 pagesNephrotic Syndrom WardHelene AlawamiNo ratings yet
- Nephrotic Syndrome in ChildrenDocument36 pagesNephrotic Syndrome in ChildrenMalueth AnguiNo ratings yet
- IVMS Cell Biology and Pathology Flash Facts 2Document3,980 pagesIVMS Cell Biology and Pathology Flash Facts 2Marc Imhotep Cray, M.D.No ratings yet
- Nephrotic Syndrome: Jaiganesh.M, M.D (General Medicine) Asst. Professor, S.M.C.HDocument60 pagesNephrotic Syndrome: Jaiganesh.M, M.D (General Medicine) Asst. Professor, S.M.C.HJaiganesh MuruganandamNo ratings yet
- Semio Renal DiaposDocument143 pagesSemio Renal DiaposMaria jose Pesantez abadNo ratings yet
- Steroid Resistant Nephrotic SyndromeDocument72 pagesSteroid Resistant Nephrotic SyndromeMonika Angra100% (1)
- Nephrotic Syndrome Children PDFDocument11 pagesNephrotic Syndrome Children PDFesdl86No ratings yet
- Diana's Renal DiseasesDocument9 pagesDiana's Renal DiseasesdhyltonNo ratings yet
- ImedclerksDocument389 pagesImedclerksWest AfricaNo ratings yet
- Attending Rounds: Fernando C. FervenzaDocument9 pagesAttending Rounds: Fernando C. FervenzaGita Christy KekenusaNo ratings yet
- Journal Reading: - Dr. Devi Albaiti Jannati - Dr. Yulita Galih PangestiDocument22 pagesJournal Reading: - Dr. Devi Albaiti Jannati - Dr. Yulita Galih PangestiDevi Albaiti JannatiNo ratings yet
- Focal Segmental GlomerulosclerosisDocument16 pagesFocal Segmental GlomerulosclerosisNagib MuhammadNo ratings yet
- Renal Pathology - 012) Nephrotic Syndrome (Notes)Document10 pagesRenal Pathology - 012) Nephrotic Syndrome (Notes)hasanatiya41No ratings yet