Professional Documents
Culture Documents
Chapter 2 Chemistry Form 6
Uploaded by
Ting TC0 ratings0% found this document useful (0 votes)
5 views10 pagespart of chap 2 from oxford
Copyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentpart of chap 2 from oxford
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
5 views10 pagesChapter 2 Chemistry Form 6
Uploaded by
Ting TCpart of chap 2 from oxford
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You are on page 1of 10
ency in the
1 Evidence of energy levels (or energy shells) in an atom is also provided by
examining the successive ionisation energies of an atom.
2 The table below lists the successive ionisation energies of the aluminium atom.
No.ofelectrons | —_onisation energy Ig
eliminated | (id mot) ionisation energy)
te aa]
_ 2745 344.~—~C*d
7 a 11578 | 4.06 |
kJ mol" 18378 = T 426 -
metas [iene ane!
EZ 7
31862 | 4.50 |
38.458 [ 458
< i‘ 42655 “4. :
_ 12 | 201 276 ees 5.30
m frequency eames) - 222313 535
1 3
3 A plot of Ig (ionisation energy) against the order of electrons ionised is plotted,
. 7
/
| '
(ionisation /
| energy)
ee eee eee
12345678 801 2 19
order of electrons removed
4 The graph shows that aluminium has 13 electrons. The successive ionisation
, proton
electron
ratio. As a result, the remaining electrons are more tightly bound by the nucleus,
energy increases because
the species produced has an ever i
There is a large increase between the third electron and the fourth electron as
well as between the eleventh and the twelfth electrons,
30 6 This indicates that the fourth electron is closer to the nucleus as compared to the
10-2) third electron. The same goes for the eleventh and twelfth electron
> Learning Outcome
Students should be able
to deduce the number
and relative energies of
the s,pand d orbitals for
the principal quantum
numbers 1, 2and 3,
including the 4s orbitals.
Firs ionisation energy of
aluminium:
Al) —> ANQ) +e
Second ionisation energy
of aluminium:
Avg) —> APM) +e
The 13th ionisation
energy of aluminium:
Dmg)
Wig) +e
Ag (ionisation energy)
is used to condense the
range ofthe ionisation
energies
‘TAKE NOTE
35
TAKE NOTE
36 Seruct
7 Hence, all the 13 electrons in aluminium can be grouped unde
[ea] et | et [nes |
Numberofelectrons | 2 | 8 3
8 The electronic configuration of aluminium is written as 2.8.3 which shows the
number of electrons in each energy shell.
The successive ionisation energy of element Q is shown below
1 314,3 388, 5 301, 7 469, 10989, 13 327,71 337, 84 080 (kJ mol")
Determine the electronic configuration of .
3a | 3388 rasa [71357 | o4080
Wireaioneeayt aie Lae [372 [ar | aoe [are | as | a92
A graph of Ig (ionisation energy) against the number of electrons ionised is plotted.
4
energy) |
+2345 678
order of electrons removed
There is a big ‘jump’ between the 6" and 7" ionisation energy. Hence, Q has 6 electrons
in the outer shell and 2 electrons in the inner shell. The electronic configuration of Q
is 26.
261
Jn the electronic configuration of aluminium, 2.8.3. How are the 3 or
1 Consider ag
8 electrons in the third shell and second shell arrang
‘A more detailed study of the line spectrum shows that the lines representing
transitions between the principal shells are in fact split into finer lines. This
the presence of subshells with different energy values in each principal
indicat
shell
antum
3. The number of subshells in a principal shell is the same as the princi
number of the principal shell
4 In any subshell, the shell with the lowest energy is represented by the letter
(which stands for sharp), followed by p (principal), d (diffuse), f fundamental),
g and so on.
NS)
Energy
4 4
Second
7 L fh
[Th 3539.34
L
am eal les
[pn 55.59.55659 | |
Further experiments on the line spectra (in the presence of a magnetic field) shows |.
that each subshell is further made up of several orbitals where the electrons are
cae Principalshel! Subset
= 6 The number of orbitals depends on the type of subshell
~ Subshell | No. of orbitals symbol | TAKE NOTE
5 1 5 zl
p 3 Py.
a 5 ty byte dg.od, |
ees me _|
The arrangement ofthe orbitals in order of inereasing energy (in an empty atom)
is as follows:
5
Bl ae
a toaorsaane
as
ja *
ns ae
0 20.
@ | =
a rr
Relative energy levels
of atomic orbitals
Us <2s<2p < 3s < 3p Learning Outcome
students should be able to
define and apply Aufbau
principle, Hund’ ule and
Pauli exclusion principle.
P21)
P1/a3
Any single orbital can
hold only 2 electrons.
“no itera spies
oT
2s ortital
18 orbital
4. The p orbital has a “dumb bell’ shape. The three p orbitals are arranged along the
x,y and z axes in space.
4 o
potbtal <-->}
shape of orbital T
‘The electronic configuration of an atom can be determined by usin:
1 The Aufbau Principle in
‘The Aufbau principle states that electrons must energy | ° 14
occupy available orbitals of lowest energy first before 2
they fill orbitals of higher energy. The diagram on the
arrangement of the orbitals in order |
right shows the
of increasing energy. (Each orbital is represented by | ¥
1 square box. The three boxes representing the three Oss ~
pp orbitals are joined, indicating that they have the 20
same energy, that is, they are degenerate orbitals Ces
1s
It is the same for the five d orbitals.)
2. Pauli Exclusion Principle
(a) The Pauli exclusion principle st
occupied by two electrons of opposite spins only
are represented by tandl.
(b) Hence, the total number of electrons in an oF
tates that each orbital (i.e. each box) can be
| The spins of the electrons
ital or set of orbitals is as follows:
[Type of orbital | Maximum no. of electrons |
od
(©) The total number of electrons that can occupy a principal shell is 2n
TAKE NOTE
ail accra eral
1 2
Zrolt cnesemts oh) =
“Tid | 3 plana Ow hank
| Fourth 4[ 2 |
Fifth s|_ 50
3 Hund’s Rule (—— aa
a
In a given set of orbitals of equivalent energy (e.g. the three p orbitals or the five | TAKENOTE
orbitals), electrons tend to occupy the orbitals singularly (with parallel spins) frst
before pairing up
For example, the two electrons in a p orbital is[1[ T]_] and not [2] |]
> Learning Outcome
flowing he es in Seton 2.8, he lestonieconiguration of the clement inthe Stlessaudbe oe
Periodic Table can be worked out. Gaiieaiantaaen
1 Hydrogen (Z = 1) has only one electron, Therefore, it will occupy the lowest eae ae
energy leveVorbital available, that is, the Ls orbital.
[1] This is written as 1s! (orb. Pues
m7
1/03, 8c
2 The electronic configuration of helium (Z=2) is se
F 7 P1/aisb
This is written as 1s* (or 2),
3 The lithium atom (Z = 3) has three electrons. The first two go into the 1s orbital,
while the third electron fills the next available empty orbital of 2s
[5] C1] tists writen as 152 or 2.4,
iF
4 Beryllium (Z = 4) has the electronic configuration of
[4] [0] this is waitten as 15225? (or 22)
7
5 Boron (Z = 5) has five electrons. The first four go into the 1s and 2s orbitals while
the fifth electron occupies one of the empty 2p orbitals.
fi) fo This is written as 1s? 2s? 2p! (or 2.3)
wer ee J
6 Caron = 6 has i elton. The 6h leon dss not oy the sme
orbital as the fifth electron, but insteads occupies one of the two remaining empty ‘
2p orbitals (Hund’s Rule).
1] @
and not
| is 2s
| @@ oto
This is written as 1s? 2s* 2p? (or 2.4)
39
Ground state
configuration
10
u
Nitrogen (Z
eRe.
7 has the electronic configuration of
‘This is written as 1s? 2s? 2p* (or 2.5)
Oxygen (Z = 8) has eight electrons. The eighth electron will have to pair up with
cone of the unpaired electrons in one of the half-filled 2p orbitals,
wo
T [4] This is writen as 1* 2% 2p! (oF 26).
(Z=18) is
Repeating the process, the electronic configuration of argo
This is written as 1s? 2s? 2p* 3s* 3p* (or 2.8.8).
The next element potassium (Z = 19) has nineteen electrons. The nineteenth
oa Gl ah
Gh) @) GE
mM
i
es
This is written as Is? 2s? 2p 3s? 3p! 4s! (or 2.8.8.1).
‘The electronic configuration of the first 36 elements is shown in the following
table. This is known as the ground state electronic configuration, that is, the
configuration with the minimum energy possible
[-Z [element [configuration | Z [Element | configuration
Cl 1s! jhe K 18°2s*2p°3s°3p"4s'
[alne [ae [20 [ca | staszprastapease
[3]u pss a1 [se Lwapssaptas
Fraenore aoe [1928 reasapaea—races |
Se fee as [v saranaeacast
| beter warer [ae le eeapseapaces |
le etwas as [vn [warner
; [slo 1828p" | 26 | Fe [re2saptsstaprscras' |
olF T [ar [co [saraparapaaes
[ho (war 2 FSrP
40
5°25%2pk3s'3p'3d4s
| 1522s%2p"3s"3p°3d%45
15:2522p35°3pr3d%45°4p"
15225'2p'38t
15228¢2p'3s'3p)
192512935939" [32 [Ge __ | 1'2s2p"3eap%adasap
ap) [a3 [as | asapraeaptadvascap’ |
[3a [se | 1s'25%apt3s%3pradeas'4p* |
35 [Br ___| tsiaszptastapradarap®
36 [kr | 1252p353p'30%4s4p |
ite Gallia
Th
12 Note that for the electronic configuration of the transition elements (Z = 21 to
Z = 30) the energy level of the 4s orbital is lower than that of the 3d orbitals,
However, once the electron/(s) is/are filled into the 3d orbital, the order is reversed.
‘The 3d orbitals now have lower energy than the 4s orbital.
(sieleheora
th
=™| ; “
I lll GET TT)
ica Ss
werefore the electronic configuration of scandium (Z = 21) is
Ist 2s? 2p* 3s? 3p® 3a" 4s? (or 2.8.9.2) and not
th Is? 2s? 2p8 3s? 3p! 4s? 3a!
The first electron ionised off from the scandium atom is from the 4s orbital and
not the 3d orbital
13. The electronic arrangement of chromium (Z= 24) is
[Ar] 36 4s! and not [Ar] 3e 4s
because the first arrangement, where the five 3d orbitals are exactly half filled, are
more stable energetically.
=) COON @ ooo ©
ud ra ad «
energetically more stable energetically less stable
14 The electronic configuration of copper (Z = 29) is
(Ar] 3d" 4s! and not [Ar] 30? 4s?
because a completely filled d subshell is more stable.
Inthe d* configuration
the electron density
Is evenly destributed
among the five 3d
orbitals
GhEET) GO)
29.1
Chop)
eet es sable
@)
*
ae
Cation electron
removal: Lastin, fist
ag
1 In the formation of cations (positive ions), electrons are removed in reverse order
from of electron filling. That is, the last electron is removed first.
2 The electronic configuration of oxygen (Z= 8) is
“&) @) Go)
2s 2p
‘The first electron to be removed is from the paired electron in the 2p orbital, Thus,
the electronic arrangement for the O* ion is
] &) Com
%p
TAKE NOTE
41
‘This is written as 1s? 2s* 2p? (or 2.5)
3. Inthe formation of anions (negative ions), electrons are added in the same manner
as the filling of electrons in the neutral atoms.
Anion electron addition:
same as neutral atoms
> 4 The electronic configuration of fluorine is ae
g
Write the electronic configuration of the following ions.
(a) AP (b) Cu © vr (@) () cl
(a) 1s? 2s? 2p (or 2.8)
(b) 1s? 28? 2p* 35° 3p" 3? (or 28.17)
(©) 18? 2s? 2p 3s? 3p" 3d? 4s! (or 2.8.1.1)
(d) 1s? 2s? 2p* (or 2.8)
(©) 1s 25° 2p* 3s? 3p* (or 2.8.8)
Emme.
1 The frequency of the first line in the Lyman series of the hydrogen atom is
2.466 x 10'S Hz. Calculate the difference in energy between the first and second
principal shells of the hydrogen atom.
2. The transition between the seventh energy level and the first energy level in an
hydrogen atom emits light with a wavelength of 9.31 x 10 m, Calculate the
difference in energy between these two levels.
3 T
10.97 1066 1052 10.27 974 a2
wave number (% 10° m-)
The Lyman series of the line spectrum of hydrogen is shown above. Calculate the 2.10.1
ionisation energy of hydrogen from the spectrum
1 1 aty
[wave number =< ctengih pa
4. Using arrows, show how the following lines in 2 Alle
the hydrogen spectrum are produced rows
(a) The third line in the Lyman series. 3 Elen
(b) The third line in the Balmer series. Be
Which of the lines have higher frequency? oe
Explain your answer.
5 Determine the maximum number of orbitals in
(a) the third principal shell,
(b) the first three principal shells of an atom.
42 r
n is
ond
the
1 In the modem Periodic Table, the elements are arranged in the order of increasing
proton numbers,
Ine Periodic Law states that the properties of elements vary periodically with
their proton numbers.
3 The modem Periodic Table has many forms. In the year 1984, the International
Union of Pure and Applied Chemistry (TUPAC) formally recommended the format
shown in below.
2.10.1
1 At present, there are 92 naturally-occurring elements. Elements with proton
numbers greater than 92 are all artificially synthesised and are radioactive.
Alll elements in the Periodic Table are grouped under 7 Periods (the horizontal
rows) and 18 Groups (the vertical columns).
3 Elements in Group 1 and Group 2 are known as the s-block elements because their
valence electrons occupy the s orbitals.
Ce of |
[Valence shell configura Ce
=am
[se Tomo [nc [usr | ate | ate ]
[Valence shetlconfiguration [2s | 38 [ae [ss] es [op
> Learning Outcome
Students should be able
(a) identity the position
‘of the elements in the
Periodic Table as
(0 lock swith valence
configurations
stands,
i) block p, with
valence shell
configurations from
Sp'tosp,
Ain Block with
valence shell
configurations from
Fs tod’s,
(b)identty the position of
elements in block fof
the Periodic Table,
rivers
[axenore
|
Thesblockemens Se
Groupe’
43
4 Elements in Group 13 (or IID) to Group
the highest orbital filled with el
elements because
18 (or VII) are known as the p-block
tron is the p orbitals
ae 3 14 5 | 16 7 | 8
a a } am | wm {of om | AS
5 mepblockelamens Element = Dst Lr fs [2 | | ‘
cele [Valence shell configuration | 33 | 3e3p% | asp | 3836 | asap" | 3530" | 2
ut eae: oo — etiet tte wes 5 PPeSaTTE NET le
5 Elements in Group 3 through Group 12 are known as the d-block elements where
ins electrons it Ives the d ort 3T
che dblock elements filling of electrons involves the d ot bitals.
—J ae ae s
eS sé 71 # [9 wine na
ement v [oe | |W | sc | az
fas | o Loe [am |e Le | | Tal) ost
Nace oe ad4s? | 34st | 34s? | 3dhas! | 34s | aehast | 30%4s! | 3chas? | 304s" | ads | 60
{configuration |" || | tt [peated aie
© eset 6 Elements with proton numbers $8 —> 71 and from 90 —> 103 are known as the
+ Pblock elements.
Proton ] aa ; ] |
a a|sfeajale a|a|s|e)a@a|e@)|e| nin ¥
[immer | Le | [m | | lo} Lo [% [ee [im |» |
eto lyspel ase esses sess tse rseursantsesmsesnseyesre | P
configuration ak oh sc
[aaccnons 7 The group number of an element indicates the number of valence electrons H
while the period number indicates the outermost principal shell that is filled with
electrons,
8 Forexample, bromine (proton number = 35) is found in Period 4 and Group 17 0f 43. ¢
the Periodic Table. Hence, its valence shell configuration is 4s° 4p" :
9 Eleven of the known elements are gases under room conditions. They are:
H,, He, Ne, Ar, Kr, Xe, Rn, N,,O,,F, and Cy
10 Only two elements are liquids at room conditions: Hg (mercury) and Br
Write the valence shell electronic configuration of the following elements.
[set [coset [roto
i 3 5
[+ 5: 7
az ‘6 4
Nx
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Analysis Past Year Chemistry SPM Question (2003-2017)Document7 pagesAnalysis Past Year Chemistry SPM Question (2003-2017)Ting TCNo ratings yet
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Periodic TableDocument8 pagesPeriodic TableKhairiyah AbdullahNo ratings yet
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- TEMPLET ReBeST SEBENAR 2019 PDFDocument1 pageTEMPLET ReBeST SEBENAR 2019 PDFTing TCNo ratings yet
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Answer, Pre-Exam Practice Chem Sem 3 EssayDocument29 pagesAnswer, Pre-Exam Practice Chem Sem 3 EssayTing TCNo ratings yet
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- EssayDocument9 pagesEssayTing TCNo ratings yet
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Kalendar STPM 2018Document2 pagesKalendar STPM 2018Ting TCNo ratings yet
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Answer For Objective QuestionsDocument20 pagesAnswer For Objective QuestionsTing TCNo ratings yet
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- A4 Sheet Labels TemplateDocument3 pagesA4 Sheet Labels TemplateEk AjnabiNo ratings yet
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Break ExsheetDocument1 pageBreak ExsheetTing TCNo ratings yet
- Shape of O3Document2 pagesShape of O3Ting TCNo ratings yet
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Skema Penang Trial SPM 2014Document21 pagesSkema Penang Trial SPM 2014Ting TCNo ratings yet
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Important Diagrams LatestDocument5 pagesImportant Diagrams LatestTing TCNo ratings yet
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Skema Penang Trial SPM 2014Document21 pagesSkema Penang Trial SPM 2014Ting TCNo ratings yet
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- New Doc 2017-07-31Document2 pagesNew Doc 2017-07-31Ting TCNo ratings yet
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Kelantan Kim K1 PDFDocument22 pagesKelantan Kim K1 PDFTing TCNo ratings yet
- Answer Scheme Term 2 TrialDocument3 pagesAnswer Scheme Term 2 TrialTing TCNo ratings yet
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Answer Scheme Term 2 TrialDocument3 pagesAnswer Scheme Term 2 TrialTing TCNo ratings yet
- SPM Physics Formula List Form 5Document0 pagesSPM Physics Formula List Form 5HaziAmiNo ratings yet
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Answer Chapter 1Document3 pagesAnswer Chapter 1Ting TCNo ratings yet
- Break SheetDocument1 pageBreak SheetTing TCNo ratings yet
- Break SheetDocument1 pageBreak SheetTing TCNo ratings yet
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- New Document ScanDocument6 pagesNew Document ScanTing TCNo ratings yet
- Break SheetDocument1 pageBreak SheetTing TCNo ratings yet
- Exp10 PDFDocument17 pagesExp10 PDFAnurag BajpaiNo ratings yet
- How To Fix Number Formatting in Mail Merge Greenwich - Computer - Help PDFDocument5 pagesHow To Fix Number Formatting in Mail Merge Greenwich - Computer - Help PDFTing TCNo ratings yet
- Break SheetDocument1 pageBreak SheetTing TCNo ratings yet
- Physical Chemistry Lab ManualDocument36 pagesPhysical Chemistry Lab ManualTing TC100% (1)
- Exp10 PDFDocument17 pagesExp10 PDFAnurag BajpaiNo ratings yet
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
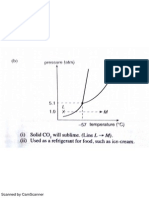
- Phase DiagramDocument3 pagesPhase DiagramTing TCNo ratings yet
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)