Professional Documents
Culture Documents
Us2656248 PDF
Uploaded by
chuckannabelleOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Us2656248 PDF
Uploaded by
chuckannabelleCopyright:
Available Formats
Patented Oct.
20, `1953
2,656,248
>,UNITED STATES PATENT OFFICE
2,656,248
AMMONIUM SULFATE PRODUCTION
Russell K. Simms, Bartlesville, Okla., assignor to
Phillips Petroleum Company, a corporation of
Delaware
Application October 21, 1949, Serial No. 122,754
4 Claims.
(Cl. 23--119)
2
of an ammonium sulfate liquor to become exces
sively acid during a process of crystallization.
Another object is to provide a process for the
This invention relates to the manufacture of
ammonium sulfate.
In one of its more specific
aspects it relates to the manufacture of improved
ammonium sulfate crystals. One embodiment of
manufacture of crystalline ammonium sulfate by
reaction of ammonia gas with sulfuric acid
wherein a plurality of evaporative crystallizers
this invention relates to an improved process for
operating two or more evaporative crystallizers
for the production of ammonium sulfate crys
tals utilizing a single separation means.
`There are many processes for the manufac
ture of ammonium sulfate, most of which are
are operated smoothly and without bumping and '
hammering caused by too great pH changes.
Other objects and advantages of this inven
tion will be apparent toA one skilled in the art
from the accompanying disclosure and discus
variations of two particular processes. The first
of these is the direct reaction of ammonia or an
ammonia-containing gas with sulfuric acid or
slon.
solutions containing same, thereby giving am
monium sulfate as product; and the second is the
gypsum process wherein calcium sulfate is re
acted with ammonium carbonate to give a prod
erating a plurality of ammonium sulfate crystal
lization units so that the pH of the liquor to be
crystallized in each unit is maintained within an
I have discovered an improved method for op
optimum range so as to produce a crystal size
in the range of 20 to 60 mesh. I have further
uctA of ammonium sulfate and a by-product of
calcium carbonate. In either of these, the am
discovered that by such control the shape and
20 character of ammonium sulfate crystals pro
monium sulfate product is recovered as an aque
ous solution and must be concentrated and evap
duced is such that their bulk density is high
and yet they do not bridge and cake on storage.
I have found that it is almost impossible to
o'rated to produce crystalline ammonium sulfate.
It is well known that ammonium sulfate crys
regulater the pH of ' a plurality of crystallizers so
tallizes in several forms, some of which are much
more advantageous than others. For example, it 25 that it remains the same for each crystallizer;
In the pH ranges dealt with in the manufacture
is known that use of considerable turbulence dur
ing the crystallization of ammonium sulfate pro
vides long needle-like crystals which have a tend
of ammonium sulfate, it has been found dii
cultto control accurately and minutely an evap
orative crystallizer utilizing a conventional pH
recorder and controller. The most desirable
range of acidity used when producing ammonium
sulfate crystals by evaporation is within the range
ency to cake and bridge on storage. This type
of crystal may also be produced when the mother
liquor, that is, an aqueous solution of ammonium
sulfate, is too acid. It is also known that fragile
and soft crystals of ammonium sulfate are usu
of 0.2 to 0.5 mol per cent excess acid which corre
sponds to a pH range of about 2.5 to 1.5. How
ever, broadly, a range of 0 to 1 mol per cent excess
acid based on ammonia may be satisfactory. I
have observed that when a group of crystallizers
ally producedwhen the mother liquor solution
from which 'the ammonium sulfate is being crys
tallized contains an excess of ammonia.
The
most advantageous type of crystals which may be
of a rhombic character which have a relatively
is operated together the optimum pH for one>
may be slightly different than that for another
high bulk density and'which generally do not
or that one may be controlled smoothly at one
cake and bridge on storage nearly as much as
the above described needle-like crystals or as
pI-l while another one may be controlled smoothly
at a slightly different pH. Further, I have foundA
produced by controlled crystallization arethose
plate-like crystals.
that operating in this particularly low range of-
It is an object of this invention to provide a
processv for the production of ammonium sulfate
pH it is diicult to control the pH Within a nar
crystals of aparticularly desirable size and shape.v
Another object is the production of ammonium
sulfate crystals of high bulk density.
Another object of this invention is to provide
content of one crystallizer may vary within a
range of from, say, almost 0 to 1 mol per cent,
or even higher at times. I have also foundthat the pH of the returned mother liquor re
a process whereby two or more evaporative crys
50
tallizers utilizing the same crystal separation
means may be operated in conjunction with one
another.
row range, and for this reason the excess acid
'
covered after separating crystals therefrom will
have considerable eiect on the smooth opera
tion of a crystallizer. For example, if a crystal
lizer is operating smoothly at an excess acid con
Still another object of this invention is to pro
tent of 0.3 mol per cent, the controlling device
vide va process for counteracting the tendency 55 will become unbalanced if a mother liquor of a
2,656,248
4
provide that which is most desirable for the par
ticular unit.
The following discussion of the drawing will
free acid content of, say, l mol per cent is in
troduced thereto.
That has actually happened
in commercial operation and a solution for this
problem is the basis for this invention. It is
of course obvious that when more than one evap
disclose in more detail one form of apparatus in
5
orative crystallizer is operating at a high acid
level the effect on the acidity of the combined
mother liquor will be cumulative and will re
quire an even longer time to correct.
It is particularly disastrous when the pI-I of the
mother liquor changes quite rapidly such as
might be caused by clogging of the acid or am
monia inlets to one of the evaporative crystal
lizers. When such a breakdown does occur,
the use of a pH controller on the mother liquor
is doubly advantageous since it prevents the pH
of the remaining crystallizers from being thrown
completely 01T.
Since the optimum free acid range for produc
ing ammonium sulfate crystals of desired size,
which my invention may be practiced, and will
give a more clear understanding of the many
aspects thereof.
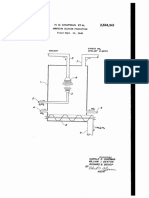
Refer now to the attached rlgure, the discus
sion of which will serve to exemplify my inven
tion. This figure shows a battery of four evapo
rative crystallizers of a modified Oslo-type piped
'as they normally are for commercial use.
Water
is passed to each of these crystallizers from line
I0 which acts as a manifold. Ammonia and sul
furic acid are passed through lines I I and I2, re
spective, in a similar manner. (It is within the
scope of my invention that a solution of am
monium sulfate be introduced to the crystallizers
which has been obtained from an outside source,
rather than producing same within the apparatus
shape, and character is in the range of 0.2 to 0.5
by the reaction of ammonia and sulfuric acid.)
mol per cent, I have found that utilization of a
Following lines II and I2, it will be noted that
pH recorder controller on the mother liquor re
they lead to the return line from the crystalliza
turned from separating apparatus such as a cen
tion zone of evaporator I. It will also be noted
trifugeV or the like, wherein crystals have been 25 that water is introduced to this same line via line
separated from mother liquor, provides optimum
I3.
control of the mother liquor Within this range
of acidity. For example, when utilizing three
evaporative crystallizers the magmas of which
are separated in a single centrifuge, the acidity
ofthe separated mother liquor may be consid
line I4, the acid reacting with the ammonia to
produce ammonium sulfate, and the water merely
erably higher than that desired for one or two
of the units. By controlling this with a pH re
corder controller which will cause to be intro
`ducedto this mother liquor a quantity of am-V monia such that the acidity is reduced to within
the desired and optimum range of 0.2 to 0.5 mol
per cent Yfree acid, the pH within the various
crystallizers will be more easily controlled to
within this range also.
40
It has been found that in changing the free
acidityof the liquor in an evaporative crystal
lizer the maximum amount of change which it is
desirable to make, as might be required when the
pH has changed undesirably due to over acidity
ofthe recycle mother liquor, is 0.1 percent in
These constituents are admixed in return
being used as a means of heat removal by its
evaporation. The ammonium sulfate liquor
which passes from line I4 into evaporator I5 is
evaporated Iby pulling a vacuum on the evapo
rator by means of condenser I6. Process water
from line I0 passes through condenser I6 there
by condensing vapor passing from evaporator .I5
through line Il. A steam ejector, not shown, is
also attached to this line to remove unconden
sable gases so that the vacuum >may be main
tained. Thus, the ammonium sulfate liquor be
comes concentrated to such ran extent that its
supersaturation passes into vthe metastable re
gion. The evaporated `and supersaturated liquor
from evaporator I5 passes downward therefrom
through line I8 into crystallizer I9 Where the
crystals therein grow by contacting the supersat
urated liquor >until they are of lthe desired size.
standpoint of smooth operation to make a change
The process fiow is so .controlled that when crys
tals become of a rdesired size (i e., in the vrange
one hour.
such as this over a period of about one-half to
Because of the considerable time re
of 20 to v60 mesh, their weight causes them to
settle through the liquor within the crystalliza
quired Vto correct pH variations it is apparent that
tion zone so that they will be withdrawn from the
fifteen minutes.
It is more desirable from the
bottom of the crystallizer as through line 2U in
the yform of a magma, which is a slurry of crys
tals and ammonium sulfate mother liquor. This
when the Ymother liquor pH was not right be
magma along with that withdrawn from similar
cause of their poor shape or other adverse char
units II. III, and IV is passed to a suitable sepa
acteris'tics.
ration means such as vcentrifuge 2 I. In this unit
-In a second embodiment of my invention I may
the `crystals are separated from the mother liquor
utilize an individual pH recorder and controller
and are washed to remove any trace of- acid
on each mother liquor return line to each of the
evaporative crystallizers rather than on the com 60 thereon. Thus, >optimum crystals >are recovered
via line -22 and `may be passed -to rotary kilns or
posite mother liquor recovered from the centri
other suitable .drying apparatus or recovery
fuge. >In this manner, the acid content of the
means.
Separated mother liquor is passed via
ammonium sulfate mother liquor being returned
the more accurate the control the better. At
times it is necessary to redissolve crystals made
line 23 to mother liquor storage tank 24. 'I'he pH
to each crystallizermay be controlled in such a
of the liquorin this tank is recorded by recorder
manner that it will conform to the particular 65
controller 25 which controls motor valve `26 on
rystallizer thus preventing unbalancing of the
ammonia inlet line 21. In this manner when the
main pH recorder controller for that crystallizer.
acid content of the liquor becomes .too high the
Also with such an arrangement the pH of a crys
recorder Acontroller opens valve 26 allowing a
tallizer which has gone beyond that desired may
quantity .of ammonia to be introduced to the liq
be more rapidly returned to within the optimum
uor thus Vreacting with V.some of the excess acid
range. In addition, when it has been found that
to produce `additional Vammonium sulfate. In
the crystallizers operate more smoothly at
addition, the controller may also be used to con
slightly .diierent p-Is the pH for the mother liq
trol the introduction of acid. Mother liquor thus
uor returned to each may be vso controlled as to 75 treated is then returned via line 28 to evaporative
2,656,248
5
crystallizer I and from line 2B through lines 29,
2. The process of claim 1 in which the pH of
30, and 38 to units II, III, and IV, respectively.
the recycled mother liquor to each of the several
Thus, it will be seen that a very adequate control
may be used to supplement recording and con
crystallization zones is separately controlled so
as to provide individual control of the pH of the
liquor in each of said zones.
3. A method of operating a series of evapo
rative crystallization zones in the production of
relatively large and uniform ammonium sulfate
trolling apparatus utilized in governing the pH
of the individual evaporative crystallization ap
paratuses.
Although this process has 1been described and
crystals, which comprises passing water, am
exemplified in terms of its preferred modifica
monia, and sulfuric acid into each of said zones
tions, it is understood that various changes may
in such proportions as to maintain therein a pH
be made without departing from the spirit and
in a range corresponding to 0.2 to 0.5 mol per
scope of the disclosure and of the claims.
cent free acid (based on ammonia) and form
-I claim:
ammonium sulfate crystals therein of a size in
1. A process for the manufacture of crystalline
ammonium sulfate in a plurality of crystalliza 15 the range of 20 to 60 mesh; withdrawing magma
from the several crystallization zones when the
tion units wherein the free acid of the liquor is
crystal size has reached said range and sepa
Yat variance from unit to unit and is to be main
rately recovering ammonium sulfate crystals and
tained within an optimum range which process
motor liquor therefrom; recycling mother liquor
comprises arranging the several crystallization
units for parallel operation so that when water, 20 to each of said zones; and as the -pH of the liquor
ammonia and sulfuric acid are introduced to said
units the free acid content in each unit is ap
proximately the same and within the range of
Y0.2 to 0.5 mol per cent excess based on ammonia,
in any one of said crystallization zones varies
from an optimum pH in said range, separately
regulating the pH of the recycled stream to that
zone so as to gradually adjust the pH of the
withdrawing and admixing the magma from 25 liquor to said optimum pH in the zone of vari
ance.
crystal formation in the several crystallization
4. The process of claim 3 in which restoration
units when the crystals have reached a size in
of optimum pH in any zone is limited to a maxi
mu-m rate of change of 0.1 per cent in 15 minutes.
RUSSELL K. SIMMS.
rately recovering ammonium sulfate crystals and 30
mother liquor in said separation zone, to assist in
References Cited in the file of this patent
keeping the free acid content in each crystallizer
UNITED STATES PATENTS
within the range of 0.2 to 0.5 mol per cent ad
justing said free acid content of recovered
Number
Name
Date
the range of 20 to 60 mesh and passing the re
sulting adrnixture to a separation zone, sepa
mother liquor to within said range, and recycling 35
the thus treated mother liquor to the various
evaporative crystallization units.
1,917,915
2,409,790
2,424,207
Atwater __________ __ July 11, 1933
Otto _____________ __ Oct. 22, 1946
Otto _____________ __ July 15, 1947
You might also like
- AlkylationDocument9 pagesAlkylationabhishek sharma100% (1)
- The Mind-Body Make OverDocument382 pagesThe Mind-Body Make OverReyaz Mohemmad100% (4)
- Phosphoric Acid Flow Sheet of Dihydrate ProcessDocument3 pagesPhosphoric Acid Flow Sheet of Dihydrate ProcessPrakash Mylar100% (1)
- Hall V Swift DismissalDocument16 pagesHall V Swift DismissalTHROnline100% (6)
- IEEE Guide For The Protection of Network TransformersDocument38 pagesIEEE Guide For The Protection of Network TransformersArmandoAguirre100% (1)
- Process Analytics in Ethylene Production PlantsDocument11 pagesProcess Analytics in Ethylene Production PlantsIka SulistyaningtiyasNo ratings yet
- Guidebook of Cwqwqhemical EngineeringDocument139 pagesGuidebook of Cwqwqhemical EngineeringMohammad FikriNo ratings yet
- GeegDocument2 pagesGeegjefferson100% (1)
- Polyaluminium ChlorideDocument8 pagesPolyaluminium ChlorideAnida MauludinaNo ratings yet
- Minimizing Corrosion in Refinery PTQDocument5 pagesMinimizing Corrosion in Refinery PTQjimbob8888No ratings yet
- Process Design For The Production of Ethylene From EthanolDocument144 pagesProcess Design For The Production of Ethylene From EthanolJorge RicoNo ratings yet
- Industrial _ Engineering Chemistry Process Design and Development Volume 19 issue 4 1980 [doi 10.1021_i260076a001] Garside, John; Shah, Mukund B. -- Crystallization Kinetics from MSMPR Crystallizers.pdfDocument6 pagesIndustrial _ Engineering Chemistry Process Design and Development Volume 19 issue 4 1980 [doi 10.1021_i260076a001] Garside, John; Shah, Mukund B. -- Crystallization Kinetics from MSMPR Crystallizers.pdfIka SulistyaningtiyasNo ratings yet
- 1st SJCL Moot Court DefendentDocument21 pages1st SJCL Moot Court Defendentsanjana seth0% (1)
- Reduce Salt Corrosion Rates With Stronger Base AminesDocument4 pagesReduce Salt Corrosion Rates With Stronger Base AminesPaolo VisentinNo ratings yet
- Sour Water Treatment UnitDocument16 pagesSour Water Treatment Unitpkgarg_iitkgp100% (2)
- The Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresFrom EverandThe Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresRating: 5 out of 5 stars5/5 (1)
- Chloride RemovalDocument12 pagesChloride Removaldilshad kapoor100% (1)
- Nobody KnowsDocument3 pagesNobody KnowsIbanElTrolazoLalalaNo ratings yet
- Sws Effect of Heat Stable SaltsDocument6 pagesSws Effect of Heat Stable SaltsEstebanNo ratings yet
- Gasoline PropertiesDocument6 pagesGasoline PropertiesbahadorNo ratings yet
- Overview Lime Slaking ProcessDocument19 pagesOverview Lime Slaking ProcessBagus Dwi UtamaNo ratings yet
- FCC Wash Water SystemsDocument16 pagesFCC Wash Water SystemsBehnam RahzaniNo ratings yet
- HRSG Supplier's Chemistry IonDocument11 pagesHRSG Supplier's Chemistry IonJoe Schroeder100% (1)
- Sour Water StrippingDocument5 pagesSour Water StrippingImam Bukhori100% (1)
- Geyser Product Catalog - March 2014Document22 pagesGeyser Product Catalog - March 2014drramsay100% (1)
- March 9, 1965 L. Mavrovic: Filed June 16, 1961Document6 pagesMarch 9, 1965 L. Mavrovic: Filed June 16, 1961baharuNo ratings yet
- Logs:: ConcentratorDocument9 pagesLogs:: ConcentratorEniNo ratings yet
- Descripsi Process Direct Neutralization Oe Synthetic ManufactureDocument9 pagesDescripsi Process Direct Neutralization Oe Synthetic Manufacturerifqi fatmalaNo ratings yet
- United States Patent 0: '3, l50, l74 ICCDocument2 pagesUnited States Patent 0: '3, l50, l74 ICCMuhammadAmdadulHoqueNo ratings yet
- Ingles CDocument8 pagesIngles Coscar rodriguezNo ratings yet
- Recent Studies On The Production of Sodium Alpha Olefin Sulfonates As Concentrates and Dry ProductsDocument10 pagesRecent Studies On The Production of Sodium Alpha Olefin Sulfonates As Concentrates and Dry Productsmushtaq521No ratings yet
- Oldest DAP PatentDocument3 pagesOldest DAP PatentVizzy VishalNo ratings yet
- Patent 01Document3 pagesPatent 01fatemeh afariNo ratings yet
- United States Patent Office: Patented June 6, 1972Document4 pagesUnited States Patent Office: Patented June 6, 1972Hector Andres CabezasNo ratings yet
- Matrix Acidizing of Sandstone4Document5 pagesMatrix Acidizing of Sandstone4HelyaNo ratings yet
- Kaasenbrood 1963Document5 pagesKaasenbrood 196325A Syifa Salsabila AlfianiNo ratings yet
- US PatentDocument4 pagesUS Patentaldo BMCNo ratings yet
- Rile From NH4Document10 pagesRile From NH4rrivera7396No ratings yet
- Us Patent Manufacture of Urea, 1954Document4 pagesUs Patent Manufacture of Urea, 195425A Syifa Salsabila AlfianiNo ratings yet
- 1912 Process of Making Precipitated Barium SulphateDocument2 pages1912 Process of Making Precipitated Barium SulphateDeluxe pNo ratings yet
- United States Patent O??ce: Patented Feb. 1, 1966Document5 pagesUnited States Patent O??ce: Patented Feb. 1, 1966Ivan AlcomendrasNo ratings yet
- 21912, Water pp170 184Document15 pages21912, Water pp170 184helloNo ratings yet
- Trace Metals in Brownstock WashingDocument27 pagesTrace Metals in Brownstock WashingSCRIBDcaroNo ratings yet
- Do Organic Amines Have A Role in The Treatment of High Purity Boiler Feedwater?Document17 pagesDo Organic Amines Have A Role in The Treatment of High Purity Boiler Feedwater?Ankit GokhaleNo ratings yet
- A THE Determination THE: SmallDocument4 pagesA THE Determination THE: SmallharulyNo ratings yet
- Polystyrene ProductionDocument4 pagesPolystyrene ProductionRishi KeshNo ratings yet
- 21 Century Phosphoric Acid Plant Designs (Bigger Is Better) : Page 1 of 8Document14 pages21 Century Phosphoric Acid Plant Designs (Bigger Is Better) : Page 1 of 8kaldjdsjkaNo ratings yet
- 10JUL07Document7 pages10JUL07Fábio Henrique Lucas da CostaNo ratings yet
- Lactic Acid Determination 1Document24 pagesLactic Acid Determination 1madads25586100% (1)
- Steam PurityDocument5 pagesSteam Puritysiruslara6491No ratings yet
- Literature SurveyDocument7 pagesLiterature SurveyVikash Sepat0% (1)
- Stacks: Ammonia Injection: A Route To CleanDocument8 pagesStacks: Ammonia Injection: A Route To CleanZEN MA100% (1)
- Boiler Feed Water and Steam ChemistryDocument4 pagesBoiler Feed Water and Steam ChemistryVajid MadathilNo ratings yet
- Us 2021699Document5 pagesUs 2021699haviedNo ratings yet
- US3232984Document6 pagesUS323298425A Syifa Salsabila AlfianiNo ratings yet
- BWT - HandbookDocument16 pagesBWT - HandbookDarko DuiloNo ratings yet
- Us Patent Process For Production of Urea, 1970Document4 pagesUs Patent Process For Production of Urea, 197025A Syifa Salsabila AlfianiNo ratings yet
- 1962 - Van Hengel - The Occurrence of InversionDocument5 pages1962 - Van Hengel - The Occurrence of InversionfelramNo ratings yet
- Polimer AnionikDocument12 pagesPolimer AnionikSandy Nugraha NugrahaNo ratings yet
- 2.0 Production of Primary Metabolites 2.1 Production of Organic Acids 2.1.1 Production of Citric AcidDocument22 pages2.0 Production of Primary Metabolites 2.1 Production of Organic Acids 2.1.1 Production of Citric AcidBharathiNo ratings yet
- Sweetening With Amonia CrystalDocument4 pagesSweetening With Amonia CrystalMohammadNo ratings yet
- US2745840 Purify SaccharinDocument3 pagesUS2745840 Purify SaccharinHerdian PebiNo ratings yet
- United States Patent Office: Patented Jan. 23, 1973Document2 pagesUnited States Patent Office: Patented Jan. 23, 1973Ahmad DawamNo ratings yet
- Carl Otto v. Koppers Company, Inc., A Delaware Corporation, and Wheeling Steel Corporation, A Delaware Corporation, 246 F.2d 789, 4th Cir. (1957)Document19 pagesCarl Otto v. Koppers Company, Inc., A Delaware Corporation, and Wheeling Steel Corporation, A Delaware Corporation, 246 F.2d 789, 4th Cir. (1957)Scribd Government DocsNo ratings yet
- III Sem ICTDocument56 pagesIII Sem ICToctoviancletusNo ratings yet
- US4780224Document4 pagesUS4780224Mohamad Reza JahanbakhshNo ratings yet
- 2000 - Smith - Some Developments in FlotationDocument4 pages2000 - Smith - Some Developments in FlotationmaithanhhaiNo ratings yet
- Sulfuric Acid TreatmentDocument9 pagesSulfuric Acid TreatmentShankar AcharNo ratings yet
- WaterDocument19 pagesWatermulldoctor1No ratings yet
- Biochemistry Applied to the Brewing Processes - Mashing, Boiling, CoolingFrom EverandBiochemistry Applied to the Brewing Processes - Mashing, Boiling, CoolingNo ratings yet
- A Further Investigation of the Symmetrical Chloride of Paranitroorthosulphobenzoic AcidFrom EverandA Further Investigation of the Symmetrical Chloride of Paranitroorthosulphobenzoic AcidNo ratings yet
- Anaerobic Co-Digestion of Algal Sludge and Waste Paper To Produce MethaneDocument5 pagesAnaerobic Co-Digestion of Algal Sludge and Waste Paper To Produce MethaneIka SulistyaningtiyasNo ratings yet
- SPSS 21 Installation - Download PDFDocument6 pagesSPSS 21 Installation - Download PDFchuckannabelleNo ratings yet
- Energy Procedia Volume 52 Issue 2014 (Doi 10.1016 - J.egypro.2014.07.089) Li, Yu-Ru Shue, Meei-Fang Hsu, Yi-Chyun Lai, Wen-Liang Chen, - Application of Factorial Design Methodology For OptimizatiDocument6 pagesEnergy Procedia Volume 52 Issue 2014 (Doi 10.1016 - J.egypro.2014.07.089) Li, Yu-Ru Shue, Meei-Fang Hsu, Yi-Chyun Lai, Wen-Liang Chen, - Application of Factorial Design Methodology For OptimizatiIka SulistyaningtiyasNo ratings yet
- Chemical Engineering Journal Volume 134 Issue 1-3 2007 (Doi 10.1016 - J.cej.2007.03.077) Abderrahim Bouaid Mercedes Martinez Jose Aracil - A Comparative Study of The Production of Ethyl Esters FromDocument7 pagesChemical Engineering Journal Volume 134 Issue 1-3 2007 (Doi 10.1016 - J.cej.2007.03.077) Abderrahim Bouaid Mercedes Martinez Jose Aracil - A Comparative Study of The Production of Ethyl Esters FromIka SulistyaningtiyasNo ratings yet
- Fuel Volume 89 Issue 1 2010 (Doi 10.1016 - J.fuel.2009.01.025) F. Ferella G. Mazziotti Di Celso I. de Michelis V. Stanisci - Optimization of The Transesterification Reaction in Biodiesel ProductiDocument7 pagesFuel Volume 89 Issue 1 2010 (Doi 10.1016 - J.fuel.2009.01.025) F. Ferella G. Mazziotti Di Celso I. de Michelis V. Stanisci - Optimization of The Transesterification Reaction in Biodiesel ProductiIka SulistyaningtiyasNo ratings yet
- Microporous and Mesoporous MaterialsDocument9 pagesMicroporous and Mesoporous MaterialsIka SulistyaningtiyasNo ratings yet
- Journal of Crystal Growth Volume 20 Issue 3 1973 (Doi 10.1016 - 0022-0248 (73) 90002-x) Maurice A. Larson John W. Mullin - Crystallization Kinetics of Ammonium SulphateDocument9 pagesJournal of Crystal Growth Volume 20 Issue 3 1973 (Doi 10.1016 - 0022-0248 (73) 90002-x) Maurice A. Larson John W. Mullin - Crystallization Kinetics of Ammonium SulphateIka SulistyaningtiyasNo ratings yet
- ITS NonDegree 16873 2308030084 Bibliography PDFDocument2 pagesITS NonDegree 16873 2308030084 Bibliography PDFchuckannabelleNo ratings yet
- Absens IDocument1 pageAbsens IchuckannabelleNo ratings yet
- Fundamentals of HPLCDocument37 pagesFundamentals of HPLCrahatulislam100% (1)
- 259 FullDocument3 pages259 FullchuckannabelleNo ratings yet
- HPLCDocument4 pagesHPLCAlbert BohrNo ratings yet
- Fundamentals of HPLCDocument37 pagesFundamentals of HPLCrahatulislam100% (1)
- Instrumental Lecture 18Document23 pagesInstrumental Lecture 18buffysangoNo ratings yet
- w365 hm2Document737 pagesw365 hm2dida100% (2)
- 2.form VI With Revision Comments PDFDocument8 pages2.form VI With Revision Comments PDFsandeep devabhaktuniNo ratings yet
- Artificial Intelligence (AI) & Intellectual Property Rights (IPR) - Legal Status and The FutureDocument3 pagesArtificial Intelligence (AI) & Intellectual Property Rights (IPR) - Legal Status and The FutureHarshit DayalNo ratings yet
- MP3 Lease License Xcaler BeatsDocument7 pagesMP3 Lease License Xcaler BeatsRahiem WilliamsNo ratings yet
- Photo Attorney "Leslie Burns" Settlement Demand LetterDocument5 pagesPhoto Attorney "Leslie Burns" Settlement Demand LetterExtortionLetterInfo.comNo ratings yet
- HFA v. Trinidad Benham - ComplaintDocument31 pagesHFA v. Trinidad Benham - ComplaintSarah BursteinNo ratings yet
- Coca Cola Group 2Document28 pagesCoca Cola Group 2Rahul SttudNo ratings yet
- Intellectual Property Cases Law DigestDocument6 pagesIntellectual Property Cases Law DigestLyien HoNo ratings yet
- Playology v. C.B. Worldwide - ComplaintDocument30 pagesPlayology v. C.B. Worldwide - ComplaintSarah BursteinNo ratings yet
- Web Copy European Partitioning Across AfricaDocument19 pagesWeb Copy European Partitioning Across Africaapi-306743033No ratings yet
- IDEN System Overview and Functional DescriptionDocument339 pagesIDEN System Overview and Functional DescriptionDavidMendezNo ratings yet
- SMB-SOL-HRM-V1.1 (Benefit)Document13 pagesSMB-SOL-HRM-V1.1 (Benefit)dilshanudayanthaNo ratings yet
- R.G Anand Vs M S. Delux Films & OrsDocument2 pagesR.G Anand Vs M S. Delux Films & Orsshiv chakrabortyNo ratings yet
- Za HL 368 Big Book Original in This Together Ver 2Document26 pagesZa HL 368 Big Book Original in This Together Ver 2Gen FiitkaNo ratings yet
- Plagiarism Submitted Manuscript Cope FlowchartDocument1 pagePlagiarism Submitted Manuscript Cope FlowchartCarlos E RinconNo ratings yet
- D7490 26723Document5 pagesD7490 26723José Feriz Torres100% (1)
- Scully U31766uDocument2 pagesScully U31766unumonveNo ratings yet
- Unit 1 Lesson 3 - WorksheetDocument10 pagesUnit 1 Lesson 3 - WorksheetmaiNo ratings yet
- PrestresedDocument2 pagesPrestresedMarcel SteoleaNo ratings yet
- Datatreasury Corporation v. Wells Fargo & Company Et Al - Document No. 733Document4 pagesDatatreasury Corporation v. Wells Fargo & Company Et Al - Document No. 733Justia.comNo ratings yet
- Healy, P.M., and Wahlen, J.M. 1999Document19 pagesHealy, P.M., and Wahlen, J.M. 1999Hidayati HasanNo ratings yet
- Baby & Childrens ProductsDocument53 pagesBaby & Childrens ProductsjaguargoldNo ratings yet











![Industrial _ Engineering Chemistry Process Design and Development Volume 19 issue 4 1980 [doi 10.1021_i260076a001] Garside, John; Shah, Mukund B. -- Crystallization Kinetics from MSMPR Crystallizers.pdf](https://imgv2-2-f.scribdassets.com/img/document/284126833/149x198/abec42366b/1444359067?v=1)