Professional Documents
Culture Documents
1A FA12 HNDT 5 Key
1A FA12 HNDT 5 Key
Uploaded by
James Wong0 ratings0% found this document useful (0 votes)
4 views2 pages1A FA12 hndt 5 key
Original Title
1A FA12 hndt 5 key
Copyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Document1A FA12 hndt 5 key
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
4 views2 pages1A FA12 HNDT 5 Key
1A FA12 HNDT 5 Key
Uploaded by
James Wong1A FA12 hndt 5 key
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You are on page 1of 2
CLAS Chem 14 Handout 5 Ch5 Gases
(5153)
1 Describe Boyle's Law, Charles’ Law and Avogadro's Law. Ifyou combine them, what do you
get?
Boyles Len) PV=constant Eom constant = R
ar Ww) PVEnRT| “ideal gas lave”
charles Lay = constant (47 VD R= 0.08206 Late
mol K
Avoppdnit law Vc cangh. (An VD
2. A tank of nitrous oxide (N,O) has a volume of 160. L and contains 34.5 kg of the gas. At 25°C,
What is the pressure of the nitrous in the tank?
Velbo &
na 24S by (LEROY TPES = POY ml
rw] +2ad = 2K
a = ner (784 mat (9-08 206 Sogo
ener pa ne OT [120 wh]
3. A balloon is filled with 1.00 L of helium at 27.0 °C and 1.00 atm, The balloon rises to a point in
the atmosphere where the temperature is -35.0 °C and the pressure is 250, torr, What is the
change in volume the balloon underwent?
2-290 bw oe 0329 ats
Vp? 7
Tye 54 23 PM = AM gen,
r = 238K Ti Wet, (el He same)
We 2M Te
TOR
Az 1.00 alw = (law) (28k)
Vy = 1:90 & CH KY (2.304 hn)
TyzMVe +4
= took - Gay
AVE Uy = 2-4 -Lou
4, Consider the flasks diagrammed below. Whats the total pressure (in atm) after the
stopcock between the two Flasks is opened? (Assume the final volume is 4.00 L)
(-00l Bool
Ye Or
0-50: 420 bor
+f
Find ner prescurt oF each eas! Prater? +
gue
fel p= Phe Oe} p= BI f
Be BY. (2Serme): = Hobe
Ve ae a
2 0.126 che > 315 foe CELERY 7 0-44 adn
5. An unknown diatomic gas has a density of 3.164 g/L at STP. What is the identity of the gas?
find molar mass of Aes STP > THoc 272k
slaty
Iv of aes is Bldg ‘
VEIL, find # miles mms 3145 aa g gle
n= PV _ (atm)(ic) 0-044G enol
a Ceti) stace Me dratomi'e | the achornre
(owg206 (AT 273K) mats of Clement is
= Q.044G mol 2 2 34S Cl s0.-
6. A mixture of 1.00 g of hydrogen and 1.00 g of helium exerts a pressure of 0.480 atm.
What is the partial pressure of each gas?
1-004 Hr (Le) = 0.5 ml Hy
Loy He Cy = 0-26 my He
nee atobte OF
Paty = Em Paap * Cone na) Paotat > (Zz Voom)
Phe > Frata~ Ft,
= 0.48 ~ 0-32 + /ON6 aha)
Pae= Xe Patel or
You might also like
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (842)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5807)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1091)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (346)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Form 1 Chapter 2 Cell As A Unit of LifeDocument5 pagesForm 1 Chapter 2 Cell As A Unit of LifeJames Wong100% (1)
- Terms and Conditions of EnrolmentDocument1 pageTerms and Conditions of EnrolmentJames WongNo ratings yet
- F2 Science James R1 F1 Science James R1 F3 Science James R1Document1 pageF2 Science James R1 F1 Science James R1 F3 Science James R1James WongNo ratings yet
- Sources of Energy: From 1 - Chapter 6Document4 pagesSources of Energy: From 1 - Chapter 6James WongNo ratings yet
- CAD Stands For: Hint: There Can Be Multiple Answers Hint: Letter Case Doesn't MatterDocument1 pageCAD Stands For: Hint: There Can Be Multiple Answers Hint: Letter Case Doesn't MatterJames WongNo ratings yet
- Financial Report Form For Taylor'S Research Grant Scheme (TRGS) School/DivisionDocument3 pagesFinancial Report Form For Taylor'S Research Grant Scheme (TRGS) School/DivisionJames WongNo ratings yet
- 1 Phang See Wang 2 Kalarani Vellasamy 3 NG Mei Peng 4 Dr. Wong Yau Siong 5 Thiagarajah Arunasalam 6 7 8 9 10 11 12Document2 pages1 Phang See Wang 2 Kalarani Vellasamy 3 NG Mei Peng 4 Dr. Wong Yau Siong 5 Thiagarajah Arunasalam 6 7 8 9 10 11 12James WongNo ratings yet
- Date Day Time Training Type Topic PaxDocument2 pagesDate Day Time Training Type Topic PaxJames WongNo ratings yet
- Aiken Format - MoodleDocsDocument3 pagesAiken Format - MoodleDocsJames Wong100% (1)
- Assessment Performance: 201608 MATH150-Class 2 (Lecture) MATH150 Pre Calculus Wong Yau HsiungDocument2 pagesAssessment Performance: 201608 MATH150-Class 2 (Lecture) MATH150 Pre Calculus Wong Yau HsiungJames WongNo ratings yet
- Spontaneous NonspontaneousDocument7 pagesSpontaneous NonspontaneousJames WongNo ratings yet
- Chapter 9 MCQs AnswerDocument6 pagesChapter 9 MCQs AnswerJames WongNo ratings yet
- Test 2 (Total 60 Marks) Name: Wong Yau Hsiung Student IDDocument7 pagesTest 2 (Total 60 Marks) Name: Wong Yau Hsiung Student IDJames WongNo ratings yet
- Test 2 Alternate AnswersDocument5 pagesTest 2 Alternate AnswersJames WongNo ratings yet
- MATH 150 Class1 PDFDocument3 pagesMATH 150 Class1 PDFJames WongNo ratings yet
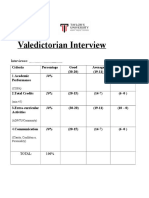
- Valedictorian Interview: IntervieweeDocument2 pagesValedictorian Interview: IntervieweeJames WongNo ratings yet
- Examiner's Report Format CHEM 105Document4 pagesExaminer's Report Format CHEM 105James WongNo ratings yet
- Assessment Performance: 201608 CHEM221-Class 1 (Lecture) CHEM221 Organic Chemistry L (With Lab) Wong Yau HsiungDocument1 pageAssessment Performance: 201608 CHEM221-Class 1 (Lecture) CHEM221 Organic Chemistry L (With Lab) Wong Yau HsiungJames WongNo ratings yet
- Assessment Performance: 201608 CHEM105-Class 4 (Lecture) CHEM105 General Chemistry I (With Lab) Wong Yau HsiungDocument2 pagesAssessment Performance: 201608 CHEM105-Class 4 (Lecture) CHEM105 General Chemistry I (With Lab) Wong Yau HsiungJames WongNo ratings yet
- Training ClaimDocument1 pageTraining ClaimJames WongNo ratings yet