0 ratings0% found this document useful (0 votes) 4K views31 pagesKinetics 3
Copyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content,
claim it here.
Available Formats
Download as DOCX, PDF or read online on Scribd
MIXED FLOW REACTOR PERFORMANCE
‘The elementary liquid-phase reaction
A+2B==R
with rate equation
mol
12.5 liter*/mol’- min)C,C ~ (15 min)Cx, [i
is to take place in a 6-liter steady-state mixed flow reactor. Two feed streams,
one containing 2.8 mol A/liter and the other containing 1.6 mol Blliter, are to
be introduced at equal volumetric flow rates into the reactor, and 75% conversion
of limiting component is desired (see Fig. E5.3). What should be the flow rate
stream? Assume a constant density throughout.
be introduced at equal volumetric low ratesinto the reactor, and 75% conversion
of limiting component is desired (see Fig. E53). What should be the flow rate
of each stream’? Assume a constant density throughout.
hon 28 mol Mlitar
{Gio= 16 no Biter
ame
v= 6lite
75% somerson ot 6
Fiore F3�SOLUTION
‘The concentration of components in the mixed feed stream is
Cyy = 14 moliliter
Cu = 08 moliliter
w= 0
‘These numbers show that B is the limiting component, so for 75% conversion
of B and « = 0, the composition in the reactor and in the exit stream is
Cy = 14 = 06/2 = 1.1 molllter
Cy = 08 ~ 06 = 02 moll
\3 mollliter
or 75% conversion
Writing the rate and solving the problem in terms of B we have at the conditions
within the reactor
Writing the rate and solving the problem in terms of B we have at the conditions
within the reactor
(=n) = RX LSNCAE} - 2X LICR
mol) (q5mol\?_ 05 ni9-1) (94m!
2s lice) (1124) (a2@2)'— caminry (oa)
—o2—_mal
Titer-min
Hence the volumentric low rate ino and out of the reactor is�For no density change, the performance equation of Eq. 13 gives
Hence the volumentric flow rate into and out of the reactor is
Ven)
Cw Co
(liter)(0.2 mot/lter- min)
(08 ~ 0.6) mol/liter
| 53. STEADY-STATE PLUG FLOW REACTOR�PLUG FLOW REACTOR PERFORMANCE
‘A homogeneous gas reaction A > 3R has a reported rate at 215°C
t= 10°CX, — [mollliter-sec]
Find the space-time needed for 80% conversion of a 50% A-S0% inert feed to
a plug flow reactor operating at 215°C and 5 atm (Cp = 0.0625 mol/iter).
Hig) 32)
Seam a
Bam ~.
igure ES4a
SOLUTION
For this stoichiometry and with 50% inerts, two volumes of feed gas would give
four volumes of completely converted product gas; thus,
4-2
in which case the plug flow performance equation, Eq. 17, becomes
rey AX, ax, C18 pos (14-X,
= Cag fae c,, fv Xa q
72 Co [A= Cw ft val wae Bee f) ax a0)
Treks
‘The integral can be evaluated in any one of three ways: graphically, numerically,
or analytically. Let us illustrate these methods.
ELOX's Conmunity�0 1 1
12
02 as" 5 1.227
04 23 1528
06 4 2
a8 9 3
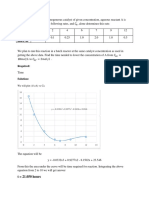
Graphical Integration. First evaluate the function to be integrated at selected
values (see Table E5.4) and plot this function (see Fig. E5.4b).
‘Average height = 1.7
‘Nea 1,7(0.8)= 1.36
0s
Xs
Figure ES
Counting squares or estimating by eye we find
AX, = (1.70)(0.8) =�Numerical Integration. Using Simpson's rule, applicable to an even number
‘of uniformly spaced intervals on the Xq axis, we find for the data of Table E54,
1s (14+ X)!2
f ( Tot) @Xq = (average height)(total width)
o WN
_ [1 + 40.227 + 201.598) +40) + 108)
| r es
106 Chapter 5 teal Reactors foro Single Reaction
Analytical Integration, From a table of integrals
14+X,)"2 LEX,
Ie) a xs
= (wen x08
7) |
= 1328
‘The method of integration recommended depends on the situation. In this prob-
lem probably the numerical method is the quickest and simplest and gives a
‘good enough answer for most purposes.
So with the integral evaluated, Eq, (i) becomes
(6.0625 mot/lites)!?
GO? motfiter'®- sex)
(133)�(ERIM r106 row neacron vou
‘The homogencous gas decomposition of phosphine
APH4(g) > P.(g) + 0H
proceeds at 649°C with the first-order rate
(10741) Cr,
What size of plug flow reactor operating at 649°C and 460 kPa can produce 80%
conversion of a feed consisting of 40 mol of pure phosphine per hour?
ho Ro 6S.
Plug iow
40moime y= 08
age
74 ~ 460 Fa
Figure ESS
SOLUTION
Let A = PH,, R = P,, § = Hy. Then the reaction becomes
4A>R+68�with
=r = (lOMhr) Cy
‘The volume of plug flow reactor is given by Eq. 21
va feareomtg aa]
Evaluating the individual terms in this expression gives
40 ml/h
k= 10/hr
Pao 460 000 Pa
T~ (&314Pa- m/mol- KY(22K
1-6,
ean toons
60 mol/m*
X, 08
hence the volume of reactor
40 mol/hr
Y= GoThe)(60 mela)
[cron -on4oy�TEST OF A KINETIC EQUATION IN A PLUG FLOW
REACTOR
We suspect that the gas reaction between A, B, and R is an elementary revers-
ible reaction
A+B==R
%
and we plan to test this with experiments in an isothermal plug flow reactor.
(a) Develop the isothermal performance equation for these kinetics for a feed
of A, B, R, and inerts.
(6) Show how to test this equation for an equimolar feed of A and B.
ELOX's Communit
108 Chapter 5 Heal Reactors for a Single Reaction
SOLUTION
(@) Feed of A, B, R, and inerts. For this elementary reaction the rate is
NyNo
VV
Ny
v
H ry = kiCaCn ~ Ce = hy
‘At constant pressure, basing expansion and conversion on substance A,
NwoXn Noo NaoXn _ 5, No + Nun,
ViL# baka) VoL + eaXa) Vo (1+ @aXa)
Letting M = Cy/Caq, M’ = Cyo/Cay We obtain
(1 = XM = Xq) _
(1+ e,Xq)
ta = iho�Hence, the design equation for plug flow, Eq. 17, becomes
(1+ eaXa)'dXq
ryiKa _
ru | 0 EyCu = RAM =X) — KAM EHNA eX)
In this expression ©, accounts for the stoichiometry and for inerts present in
the feed.
(b) Equimolar feed of A and B. For Cy = Cao, Cy = 0, and no inerts, we
have M = 1, M’ = 0,6, = ~0.5;hence the expression for part a reduces to
yy (1-05%,F dX, ‘ell [hy
1 Rea HFK = 08K) ae Jireoex,
Having V, vo, and X,, data from a series of experiments, separately evaluate
the left side and the right side of Eq. (i). For the right side, at various XY,
: °
zs
Se
is (0 pedis a
aif sro ine
7 Wep
Figure E56
2 guemayroue rung rium meucivn awe
evaluate f(X,), then integrate graphically to give Jf(Xq)dXy and then make
the plot of Fig. ES6. If the data fall on a reasonably straight line, then the
suggested kinetic scheme can be said to be satisfactory in that it fits the data
5.1. Consider a gas-phase reaction 2A —> R + 2 with unknown kinetics. If a
space velocity of Limin is needed for 90% conversion of A in a plug flow
reactor, find the corresponding space-time and mean residence time or
holding time of Muid in the plug low reactor.�Bl By the definition
Vote thet bxouiag =
eee teh GH) te Ent
etua e
5.3. A stream of aqueous monomer A (1 moVfiter, 4 liter/min) enters a 2-titer
mixed flow reactor, is radiated therein, and polymerizes as follows:
ASR s—S 7,
In the exit stream C, = 0.01 mollliter, and for a particular reaction product
W. Cw = 0.0002 moliter. Find the rate of reaction of A and the rate of
formation of W.�. An aqueous feed of A and B (400 liter/min, 100 mmol Alliter, 200 mmol
Biliter) is to be converted to product in a plug flow reactor. The kinetics
of the reaction is represented by
AS BR, 1 =200C,C, 701
Titer-min
Find the volume of reactor needed for 99.9% conversion of A to product.
5.7. The off gas from a boiling water nuclear power reactor contains a whole
variety of radioactive trash, one of the most troublesome being Xe-133,
(half life = 5.2 days). This off gas flows continuously through a large holdup
tank in which its mean residence time is 30 days, and where we can assume
that the contents are well mixed. Find the fraction of activity removed in
the tank.
59. A specific enzyme acts as catalyst in the fermentation of reactant A. At a
given enzyme concentration in the aqueous feed stream (25 liter/min) find
the volume of plug flow reactor needed for 95% conversion of reactant
A (Cxy = 2 molliter). The kinetics of the fermentation at this enzyme
concentration is given by
je pea ley __mol
®~ 7405 C, liter-min�SL. Enzyme E catalyses the fermentation of substrate A (the reactant) to
product R. Find the size of mixed flow reactor needed for 95% conversion,
of reactant in a feed stream (25 liter/min) of reactant (2 mol/liter) and
enzyme. The kinetics of the fermentation at this enzyme concentration are
given by
_ 1, __mol
"ST405C, liter mi
5.13. At 650°C phosphine vapor decomposes as follows:
APH, + P(g) + Hy, Fa = (LD HC a
What size of plug flow reactor operating at 649°C and L1.4 aim is needed
for 75% conversion of 10 moVhr of phosphine in a 2/3 phosphine-1/3
inert feed?�5.15, A gascous feed of pure A (1 molliter) enters a mixed flow reactor (2 liters)
and reacts a follows:
2ASR, r= 005, —2
ier: sec
Find what feed rate (liter/min) will give an outlet concentration Cy = 0.5
molliter,
S.I7. 1 liter/s of a 20% ozone-80% air mixture at 1.5 atm and 93°C passes
through a plug flow resctor. Under these conditions ozone decomposes by
homogeneous reaction
203303, —rewoe = KChones k= 05 ter
‘What size reactor is needed for 50% decomposition of ozone? This problem
is a modification of a problem given by Corcoran and Lacey (1970).�5.19. Pure gaseous A at about 3 atm and 30°C (120 mmollliter) is fed into a 1-
liter mixed flow reactor at various flow rates. There it decomposes, and
the exit concentration of A is measured for each flow rate. From the
following data finda rate equation torepresent the kinetics of the decompo-
sition of A. Assume that reactant A alone affects the rate.
iter/min | 006 048 1S 81
tos ABR
Cyymmovliter | 30 60 80�‘S21 We are planning to operate a batch reactor to convert A into R. This is ¢
liquid reaction, the stoichiometry is A->R, and the rate of reaction is
given in Table P5.21. How long must we react each batch for the concentra.
tion to drop from C,) = 1.3 mollliter to C4y = 0.3 molfiter?
‘Table PS.21
Cay moliliter ray mol/liter min
1 on
02 03
03 os
oF 06
os os
06 025
o7 0.10
o8 006
10 0s
13 0045
20 0042
521 the approach which probably fiat comes to rmind & te finda
rate eqvahin fo repr the date, ft onder, second, ete
iF to ge the tie; os ——
pases AS
edata. shows thak ne smple
Bok a quik plot of A
rate forn er ® will Al the data, s
cy
With a Wik more thought we coe that we stirs nah ated to fl
rake equahin,wa were {ust aclead for t, and that th
pole Yi olan by aot
jemed dlemgy aqachin Sy
dwacty by qraphca yrotecluyes, het vs clo
anf ma * henna 2�5.23, (a) For the reaction of Problem 521, what size of mixed flow reactor is
needed for 75% conversion of a feed stream of 1000 mol A/hr at Cay
.2 molliter?
(b) Repeat part (a) with the modification that the feed rate is doubled,
thus 2000 mol A/hr at Cy) = 1.2 moliiter are to be treated.
(©) Repeat part (a) with the modification that C,y = 2.4 moVliter; however,
1000 mol A/hr ate still to be treated down to Cy = 03 moliter.�525. The aqueous decompesition of A is studied in an experimental mixed flow
reactor. The results in Table P5.25 are obtained in steady-state runs. To
obtain 75% conversion of reactant in a feed, Cay = 0.8 mollliter, what
holding time is needed in a plug flow reactor?
Table P5.25
Concentration of A, moVliter_ Holding Time,
In Exit Stream sec
0.65 300
os 240
1.00 250
056 110
1.00 om 360
0.48 oan 24
048 028 200
048 0.20 560�125 for a inal flow Teackor, with GO, we find the tate of
teachin. From Eq ( This we talvlate ax follows
on
2 doe
oat 1000
ate 1500
Halding hime. for gli Plnes Fine
v 4208} Gros
rag mes by aig emvrning oo WSR
wit the tat Whee oe ro Cont
the ve of concenrahons gates no praens
Lat us use this, Then he performance expression is Ey oF
re- (“3
So lataate 4 nit oe tale above
Nowa plot Bf Chee =Ty ques a fore bke diss | /
is mdicaes that no simple rate exprestion,say 7
wh order, wil fF te doo, here abe grep
This is done by patsy Gos Mem) ond mtegmtng
ig fle rant
Pot = 200 oe eg
5.27. HOLMES: You say he was last seen tending this vat .
SIR BOSS: You mean “overflow stirred tank reactor,” Mr. Holmes.
HOLMES: You must excuse my ignorance of your particular technical
jargon, Sir Boss.
SIR BOSS: That's all right: however, you must find him, Mr. Holmes.�Imbibit was a queer chap; always staring into the reactor, taking deep
breaths, and licking his lips, but he was our very best operator. Why,
since he left, our conversion of googliox has dropped from 80% to 75%.
HOLMES (tapping the side of the vat idly): By the way, what goes on in
the vat?
SIR BOSS: Just an elementary second-order reaction, between ethanol
and googliox, if you know what I mean. Of course, we maintain a large
excess of alcohol, about 100 to 1 and
HOLMES (interrupting): Intriguing, we checked every possible lead in
town and found not a single clue.
SIR BOSS (wiping away the tears): We'll give the old chap a raise—about
twopence per week—if only he'll come back
DR. WATSON: Pardon me, but may Task a question?
HOLMES: Why certainly, Watson,
WATSON: What is the capacity of this vat, Sir Boss?
SIR BOSS: A hundred Imperial gallons, and we always keep it filled to
the brim. That is why we call it an overflow reactor. You see we are
running at full capacity—profitable operation you know.
HOLMES: Well, my dear Watson, we must admit that we're stumped, for
without clues deductive powers are of no avail.
WATSON: Ahi, but there is where you are wrong, Holmes. (Then, turning
{o the manager): Imbibit was a largish fellow—say about 18 stone—was
he not?
SIR BOSS: Why yes, how did you know?
HOLMES (with awe): Amazing, my dear Watson!
WATSON (modestly): Why it’s quite elementary, Holmes. We have all the
clues necessary to deduce what happened to the happy fellow. But first
of all, would someone fetch me some dill?
With Sherlock Holmes and Sir Boss impatiently waiting, Dr. Watson
casually leaned against the vat, slowly and carefully filed his pipe, and—
with the keen sense of the dramatic—it it. There cur story ends,
(a) What momentous revelation was Dr. Watson planning to make, and
how did he arrive at this conclusion?
(b) Why did he never make it?�Sty Since the vat codous ~14% ethane! | ques that Iwloibit
fell—dead drune— mtv the vat Ther woud decrease the
yolome aveitable fr flid. Lets see’ aie eoald account
for the decteass is conversion of goegiion
Cewordly, cmce ethanol 1S 4 Voy faige exces We can
venconably assume peewse Firar order Evietes tot tecgect te
Seager > Se Be tmed loo Eqt® gues, forbove
Ha
bY, = a
Naf 7h we hove
Wy =100 (HITE Imp gat
Sethe decwaco'n wlvne ie AY = \oo-7E = 2E (up ga!
Gs od (TENS) Gaver) = 42.8?
Ua sn oon'
ABS yolumas bith his every
berg C2r0/A
Wmbibts vole ts
“hese obmes qgtee co Init auld wall beta he sat: }
—
This hypothesis, guese doce At the facts
b thy did Watson never come up tith Has explanation?
Eeause tweaking near He alhel tae Net really coal <——
Hole + Ween was: indt kainate on peklng. Yeo ose dal
for prting m bine, net ckauol
+ Of costse euerjone kamvos shat { lag gah Wa velome
BE ICH MSO, ara chat {aime waghe ot�$28 The data in Table P5.28 have been obtained on the decomposition of
gaseous reactant A in a constant volume batch reactor at 100°C. The
Table P5.28
see Pay atm 1 90e Pay atm
0 1.00 140 025
20 080 200 oa
40 0.68 260 0.08
60 056 330 0.08
80 04s 420 0.02
100 037
ELOK’s Community.
Problems 119
stoichiometry of the reaction is 2A -* R + S. What size plug flow reactor
(in liters) operating at 100°C and 1 atm can treat 100 mol A/hr in a feed
consisting of 20% inerts to obtain 95% converson of A?
5.29, Repeat the previous problem for a mixed flow reactor.�B2Y Cegtension OF problem 5-28)
Given a goe phase reackon ZA=R4+S
M2
Liat ‘
Cpe t00 mone —— Senivone
Avo works infeed
1. Bas =100(2) «125 0tYnr —— Flmabe gen
fe sate Tyg2 Hehe
Far med Flow we must Sus the cate at the ont
eomdtione then tie enn procerd to Puecbing tha tenctor
ye Sodhaw ar accuvahe fj oe t ease Gud find
Hee stage Chevee vate) of fu~ Ootat
t
aats, 208, £0 sbw,
raleslope = Goo = SES ty
Te ate
The performace expiahim Sr cimed flow, Eq \%, on
precewse nite ie
eM 2 feof. 08-04
tr” Catfvod abel.
Rut Tall so evauate ov fiom per=nRT Thee
wat &T _ (25 Ko0eret
P ay
“y
= 21504 = 21S�| Example 3-7. Determining C; = hy(X) for a Gas-Phase Reaction
‘A mixture of 285 SO, and 72% air is charged to a low reactor in which SO, is
oxidized,
280,+0, ——+ 280,
First, set up a stoichiometric tble using only the symbols (Le, @,, F;) and then
‘prepare a second stoichiometric table evaluating aumerically as many symbols as
[Possible for the case when the fotal pressure is [485 kPa and the temperature is con-
stant 227°C,
Solution
‘Taking SO, as the basis of calculation, we divide the reaction through by the sto-
ichiometrc coefficient of our chosen basi of calculation
30,440; — 50,
‘The inal stichiorsewic tuble is given as Table E3-7.1, Inilly, 72% of the total
umber of moles is sir contaicing 21% 0, and 79% N,.
Fro = (028) Froh
0.72021) Fx)
072.021) _ 4.54
028
= Fe _ (072)(079) _
OnE, 028 72°
‘To write concentration in terms of conversion, we must express the volumetric Low
rate as a fuuction of conversion.
FLY 80,
mV; 40
‘Therefore,�Liquid Phase Gas Phase
xosenpemsteMentrnes
venaoa( YE)
Cu(0 2x
Figure 3.3. Expressing concentation as a function of conversion,�Fy _ Fag X)
a= 7
Recalling Equation (3-44), we have
=wuren (2)
a
Neglecting pressure drop in the reacion, P= Py, yields
tent
voit ven E
Tf the reaction is also carried out isothermaily, T= T,, we obtain
v= ettex)
= Full=X)
uy ex)
"s ©
=x
Trex,
‘The concentration ofA initially is equal to the mole fraction ofA intially multiplied
by the total concentration. The total concentration can te calculated from an equa
tion of state such as the ideal gas law:
00 Gia ene SE)
= 01 movi?
“The total concentration is�= = =
natn totiny Ons
7 ce em
Se
‘ig ca
sagan an�rane 8 Calin gris Cone
Burret nr toro sp 0, ae
0, a0,
‘Ra ASO Tecoma boas tna 0H SOT
pat,
(a Eaten serie o 04
0) GiSinte itsim omesone NO, at ete
| Setvemiceemerermeretmme
— i
cin oot mag cet oe ace |
‘SSUES etme Ae '
esd
‘
Re eo eT
a Pee ean
chet cca ou
eal et was
a oan
Can aE” Ta en pel TRS
feel 1= 3 nl wens in 42) 4
sate
1 ES
‘eo UN ne ep oeion nd he
(Bie ston CYR ome Seamer
=e pense) +405
‘he FOLYMATH pop nd ta ies 2 a KS
‘Recut omen coms a
ote�uae 3) Me em ef ot Fie ats
coir merry ion Sd Bap
No, 280,
| Senet eee remit eaiemsie
Mae Bon wr
Son ea
ar
~xeufel
all ess,
eee ao i hn
ok m9
Cong Enos ES) a 8.63 win
yi
1�asian)
00)
a ol Sa) sate 05
— ene
ie, ie i Egon 2-19) 8 Epon 010 an aie
Sociee
caneufeafa} as
|
|
|�‘Sj ce G01) we deen Cy dC, .
cabs FAM as eycrma eA eosein
AO as HytT=aeg "MES 8
ce Be SFA aioe
oP araiviaas SOI d tee IE
seme eee
cnaasicoon ER | ein
RES
n= ate IS“ MASTS FLS— BY DIO)
Meld mow me oD
Fe = Fa Fofa™ Fel 786-058)
fa onto et Dk a te wi oe
You might also like
Given A Dilute Aqueous Feed, C C 100, A+2B R+S, C 20. Find X, X, C
Given A Dilute Aqueous Feed, C C 100, A+2B R+S, C 20. Find X, X, C
2 pages