Professional Documents
Culture Documents
Nej M 200001273420407
Uploaded by
Tony Gomez Luna LeyvaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Nej M 200001273420407
Uploaded by
Tony Gomez Luna LeyvaCopyright:
Available Formats
The Ne w E n g l a nd Jo u r n a l o f Me d ic i ne
Review Article
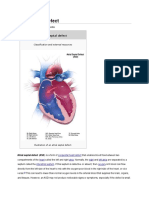
Medical Progress tial anomalous drainage of the pulmonary veins into
the right atrium or venae cavae (with sinus venosus
defects).5 Although most atrial septal defects result
from spontaneous genetic mutations, some are in-
C ONGENITAL H EART D ISEASE herited.6-9
IN A DULTS Regardless of anatomical location, the physiologic
consequences of atrial septal defects are the result of
the shunting of blood from one atrium to the other;
First of Two Parts the direction and magnitude of shunting are deter-
mined by the size of the defect and the relative com-
M. ELIZABETH BRICKNER, M.D., L. DAVID HILLIS, M.D., pliance of the ventricles. A small defect (less than 0.5
AND RICHARD A. LANGE, M.D. cm in diameter) is associated with a small shunt and
no hemodynamic sequelae. A sizable defect (more
than 2 cm in diameter) may be associated with a large
shunt, with substantial hemodynamic consequences.
O VER the past 20 to 30 years, major advanc- In most adults with atrial septal defects, the right
es have been made in the diagnosis and treat- ventricle is more compliant than the left; as a result,
ment of congenital heart disease in children. left atrial blood is shunted to the right atrium, caus-
As a result, many children with such disease now sur- ing increased pulmonary blood flow and dilatation of
vive to adulthood. In the United States alone, the the atria, right ventricle, and pulmonary arteries (Fig.
population of adults with congenital heart disease, ei- 1). Eventually, if the right ventricle fails or its com-
ther surgically corrected or uncorrected, is estimated pliance declines, the left-to-right shunting diminishes
to be increasing at a rate of about 5 percent per year; in magnitude, and right-to-left shunting may even
this year there will be almost 1 million such patients.1 occur.
This two-part review discusses the more common In a patient with a large atrial septal defect, a right
acyanotic and cyanotic congenital heart conditions ventricular or pulmonary arterial impulse may be pal-
that physicians who care for adults are likely to en- pable. The first heart sound is normal, and there is
counter. wide and fixed splitting of the second heart sound.
The splitting of the second heart sound is fixed be-
ACYANOTIC CONDITIONS cause phasic changes in systemic venous return to the
Atrial Septal Defect right atrium during respiration are accompanied by
Atrial septal defect accounts for about one third of reciprocal changes in the volume of shunted blood
the cases of congenital heart disease detected in adults. from the left atrium to the right atrium, thereby min-
It occurs in women two to three times as often as in imizing the respiratory changes in right and left ven-
men.2,3 Anatomically, it may take the form of ostium tricular stroke volumes that are normally responsible
secundum, in the region of the fossa ovalis; ostium for physiologic splitting.10 A systolic ejection murmur,
primum, in the lower part of the atrial septum; or audible in the second left intercostal space, peaks in
sinus venosus, in the upper atrial septum. Ostium se- mid-systole, ends before the second heart sound, and
cundum defects make up 75 percent of all atrial sep- is usually so soft that it is mistaken for an innocent
tal defects, ostium primum defects make up 15 per- flow murmur. Flow across the atrial septal defect it-
cent, and sinus venosus defects make up 10 percent. self does not produce a murmur.
Additional cardiac abnormalities may occur with each Electrocardiographically, a patient with atrial sep-
type of defect; these include mitral-valve prolapse tal defect often has right-axis deviation and incom-
(with ostium secundum defects),4 mitral regurgitation plete right bundle-branch block. Left-axis deviation
(due to a cleft in the anterior mitral-valve leaflet, occurs with ostium primum defects. A junctional or
which occurs with ostium primum defects), and par- low atrial rhythm (inverted P waves in the inferior
leads) occurs with sinus venosus defects. A patient
with an atrial septal defect usually has normal sinus
rhythm for the first three decades of life, after which
From the Department of Internal Medicine, Cardiovascular Division, atrial arrhythmias, including atrial fibrillation and
University of Texas Southwestern Medical Center, Dallas. Address reprint supraventricular tachycardia, may appear.11 On chest
requests to Dr. Hillis at Rm. CS7.102, University of Texas Southwestern
Medical Center, 5323 Harry Hines Blvd., Dallas, TX 75235-9047. radiography, the patient has prominent pulmonary
2000, Massachusetts Medical Society. arteries and a peripheral pulmonary vascular pattern
256 Ja nu ar y 2 7 , 2 0 0 0
The New England Journal of Medicine
Downloaded from nejm.org at UNIVERSIDAD NACIONAL AUTONOMA DE MEXICO on May 21, 2017. For personal use only. No other uses without permission.
Copyright 2000 Massachusetts Medical Society. All rights reserved.
MED IC A L PROGR ES S
toms and are not accompanied by striking abnormal-
ities on physical examination, they often remain un-
Pulmonary artery detected for years.3,13,14 A small defect with minimal
left-to-right shunting (characterized by a ratio of pul-
Atrial septal-
defect
monary to systemic flow of less than 1.5) usually caus-
es no symptoms or hemodynamic abnormalities and
Pulmonary- therefore does not require closure. Patients with mod-
veins erate or large atrial septal defects often have no symp-
toms until the third or fourth decades of life despite
Left" substantial left-to-right shunting (characterized by a
atrium ratio of pulmonary to systemic flow of 1.5 or more).
Over the years, the increased volume of blood flowing
through the chambers of the right side of the heart
Right"
" usually causes right ventricular dilatation and fail-
atrium ure.3,14-16 Obstructive pulmonary vascular disease (Ei-
senmengers syndrome) occurs rarely in adults with
atrial septal defect.14
A symptomatic patient with an atrial septal defect
Left"
ventricle typically reports fatigue or dyspnea on exertion. Alter-
natively, the development of such sequelae as supra-
Right" ventricular arrhythmias, right heart failure, paradoxi-
ventricle cal embolism, or recurrent pulmonary infections may
prompt the patient to seek medical attention. Al-
though a few patients with an unrepaired atrial septal
defect have survived into the eighth or ninth decade of
life,17 those with sizable shunts often die of right ven-
tricular failure or arrhythmia in their 30s or 40s.3,13-15
An atrial septal defect with a ratio of pulmonary
Figure 1. Atrial Septal Defect with Resultant Left-to-Right to systemic flow of 1.5 or more should be closed sur-
Shunting.
gically to prevent right ventricular dysfunction.18-20
Blood from the pulmonary veins enters the left atrium, after
which some of it crosses the atrial septal defect into the right
Surgical closure is not recommended for patients with
atrium and ventricle (longer arrow). irreversible pulmonary vascular disease and pulmo-
nary hypertension.21 Although devices for percutane-
ous atrial septal closure are under investigation,22,23
their safety and efficacy are unknown. Prophylaxis
of shunt vascularity (in which the small pulmo- against infective endocarditis is not recommended
nary arteries are especially well visualized in the pe- for patients with atrial septal defects (repaired or un-
riphery of both lungs). repaired) unless a concomitant valvular abnormality
Transthoracic echocardiography may reveal dilata- (e.g., mitral-valve cleft or prolapse) is present.24
tion of the atria and right ventricle. Ostium primum
or ostium secundum defects are often visualized di- Ventricular Septal Defect
rectly, but transthoracic echocardiography usually does Ventricular septal defect is the most common con-
not identify sinus venosus defects.12 The sensitivity of genital cardiac abnormality in infants and children. It
echocardiography may be enhanced by injecting mi- occurs with similar frequency in boys and girls. Twen-
crobubbles of air in solution into a peripheral vein, ty-five to 40 percent of such defects close spontane-
after which the movement of some of the bubbles ously by the time the child is 2 years old; 90 percent
across the defect into the left atrium can be visual- of those that eventually close do so by the time the
ized. Transesophageal and Doppler color-flow echo- child is 10.11,25 Anatomically, 70 percent are located in
cardiography are particularly useful in detecting and the membranous portion of the interventricular sep-
determining the location of atrial septal defects and tum, 20 percent in the muscular portion of the sep-
in identifying sinus venosus defects and anomalous tum, 5 percent just below the aortic valve (thereby
pulmonary venous drainage. Although echocardiog- undermining the valve annulus and causing regurgi-
raphy may provide enough information to guide the tation), and 5 percent near the junction of the mitral
management of an atrial septal defect, catheterization and tricuspid valves (so-called atrioventricular canal
may be required to determine the magnitude and di- defects).26
rection of shunting, as well as whether pulmonary hy- The physiologic consequences of a ventricular sep-
pertension is present and, if so, its severity. tal defect are determined by the size of the defect
Since atrial septal defects initially produce no symp- and the relative resistance in the systemic and pul-
Vol ume 342 Numb e r 4 257
The New England Journal of Medicine
Downloaded from nejm.org at UNIVERSIDAD NACIONAL AUTONOMA DE MEXICO on May 21, 2017. For personal use only. No other uses without permission.
Copyright 2000 Massachusetts Medical Society. All rights reserved.
The Ne w E n g l a nd Jo u r n a l o f Me d ic i ne
(caused by increased flow through the mitral valve)
may be heard, and a decrescendo diastolic murmur of
Pulmonary artery
aortic regurgitation may be present if the ventricular
septal defect undermines the valve annulus. Small,
muscular ventricular septal defects may produce high-
frequency systolic ejection murmurs that terminate
before the end of systole (when the defect is occlud-
ed by contracting heart muscle). If pulmonary hyper-
tension develops, a right ventricular heave and a pul-
Left"
sation over the pulmonary trunk may be palpated. The
atrium holosystolic murmur and thrill diminish and eventu-
ally disappear as flow through the defect decreases,
and a murmur of pulmonary regurgitation (Graham
Right" Steells murmur) may appear. Finally, cyanosis and
"
atrium clubbing are present.
Electrocardiography and chest radiography provide
insight into the magnitude of the hemodynamic im-
pairment. With a small ventricular septal defect, both
Left"
ventricle
are normal. With a large defect, there is electrocardio-
graphic evidence of left atrial and ventricular enlarge-
ment, and left ventricular enlargement and shunt vas-
Right" cularity are evident on the radiograph. If pulmonary
ventricle hypertension occurs, the QRS axis shifts to the right,
and right atrial and ventricular enlargement are noted
on the electrocardiogram. The chest film of a patient
Ventricular- with pulmonary hypertension shows marked enlarge-
septal defect ment of the proximal pulmonary arteries, rapid ta-
pering of the peripheral pulmonary arteries, and oli-
gemic lung fields. Two-dimensional echocardiography
Figure 2. Ventricular Septal Defect with Resultant Left-to-Right with Doppler flow can confirm the presence and lo-
Shunting. cation of the ventricular septal defect, and color-flow
When the left ventricle contracts, it ejects some blood into the mapping provides information about the magnitude
aorta and some across the ventricular septal defect into the
right ventricle and pulmonary artery (arrow).
and direction of shunting.27,28 With catheterization
and angiography, one can confirm the presence and
location of the defect, as well as determine the mag-
nitude of shunting and the pulmonary vascular resist-
monary vascular beds. If the defect is small, there is ance.29
little or no functional disturbance, since pulmonary The natural history of ventricular septal defect de-
blood flow is increased only minimally. In contrast, pends on the size of the defect and the pulmonary
if the defect is large, the ventricular systolic pressures vascular resistance. Adults with small defects and nor-
are equal and the magnitude of flow to the pulmo- mal pulmonary arterial pressure are generally asymp-
nary and systemic circulations is determined by the re- tomatic, and pulmonary vascular disease is unlikely to
sistances in the two beds. Initially, systemic vascular re- develop.30 Such patients do not require surgical clo-
sistance exceeds pulmonary vascular resistance, so that sure, but they are at risk for infective endocarditis31,32
left-to-right shunting predominates (Fig. 2). Over and should therefore receive antibiotic prophylaxis.
time, the pulmonary vascular resistance usually in- In contrast, patients with large defects who survive
creases, and the magnitude of left-to-right shunting to adulthood usually have left ventricular failure or
declines. Eventually, the pulmonary vascular resistance pulmonary hypertension with associated right ven-
equals or exceeds the systemic resistance; the shunting tricular failure.11 Surgical closure of the defect is rec-
of blood from left to right then ceases, and right-to- ommended, if the magnitude of pulmonary vascular
left shunting begins. obstructive disease is not prohibitive. Once the ratio
With substantial left-to-right shunting and little or of pulmonary to systemic vascular resistance exceeds
no pulmonary hypertension, the left ventricular im- 0.7, the risk associated with surgery is prohibitive.
pulse is dynamic and laterally displaced, and the right
ventricular impulse is weak. The murmur of a mod- Patent Ductus Arteriosus
erate or large defect is holosystolic, loudest at the low- The ductus arteriosus connects the descending aor-
er left sternal border, and usually accompanied by a ta (just distal to the left subclavian artery) to the left
palpable thrill. A short mid-diastolic apical rumble pulmonary artery. In the fetus, it permits pulmonary
258 Ja nu ar y 2 7 , 2 0 0 0
The New England Journal of Medicine
Downloaded from nejm.org at UNIVERSIDAD NACIONAL AUTONOMA DE MEXICO on May 21, 2017. For personal use only. No other uses without permission.
Copyright 2000 Massachusetts Medical Society. All rights reserved.
MED ICA L PROGR ES S
and aortic valves, respectively) may be noted. If pul-
Ductus arteriosus monary vascular obstruction and hypertension de-
Aorta velop, the continuous murmur decreases in duration
and intensity and eventually disappears and a pulmo-
nary ejection click and a diastolic decrescendo mur-
mur of pulmonary regurgitation may appear.
With a small patent ductus arteriosus, the electro-
Pulmonary-
cardiogram and chest x-ray film are normal. With a
artery large patent ductus arteriosus and substantial left-to-
right shunting, left atrial and ventricular hypertro-
Left" phy are evident, and the chest film shows pulmonary
atrium plethora, proximal pulmonary arterial dilatation, and a
prominent ascending aorta. The ductus arteriosus may
be visualized as an opacity at the confluence of the
Right"
" descending aorta and the aortic knob. If pulmonary
atrium hypertension develops, right ventricular hypertrophy
is noted. With two-dimensional echocardiography,
Left"
ventricle
the ductus arteriosus can usually be visualized, and
Doppler studies demonstrate continuous flow in the
pulmonary trunk.33 Catheterization and angiography
make it possible to quantify the magnitude of shunt-
Right" ing and the pulmonary vascular resistance as well as
ventricle visualize the ductus arteriosus.29
A patent ductus arteriosus rarely closes spontane-
ously after infancy.34 A small patent ductus arteriosus
causes no symptoms, and a person with a defect of
this size can have a normal life expectancy. However,
the presence of a small patent ductus arteriosus en-
tails an elevated risk of infective endocarditis, which
Figure 3. Patent Ductus Arteriosus with Resultant Left-to-Right
Shunting.
involves the pulmonary side of the ductus arteriosus or
Some of the blood from the aorta crosses the ductus arteriosus
the pulmonary artery opposite the duct orifice, from
and flows into the pulmonary artery (arrows). which septic pulmonary emboli may arise. A patent
ductus arteriosus of moderate size may cause no symp-
toms during infancy; during childhood or adulthood,
fatigue, dyspnea, or palpitations may appear.35,36 In ad-
arterial blood to bypass the unexpanded lungs and dition, the ductus arteriosus may become aneurys-
enter the descending aorta for oxygenation in the mal and calcified, which may lead to its rupture.37-40
placenta. It normally closes soon after birth, but in With larger shunts, flow is markedly increased, which
some infants it does not close spontaneously, and may precipitate left ventricular failure. Eventually, pul-
there is continuous flow from the aorta to the pul- monary vascular obstruction may develop; when the
monary artery (i.e., left-to-right shunting) (Fig. 3). pulmonary vascular resistance equals or exceeds the
Patent ductus arteriosus accounts for about 10 per- systemic vascular resistance, the direction of shunting
cent of cases of congenital heart disease. Its incidence reverses.35,41 One third of patients with a patent duc-
is higher than average in pregnancies complicated by tus arteriosus that is not surgically repaired die of
persistent perinatal hypoxemia or maternal rubella heart failure, pulmonary hypertension, or endarteri-
infection and among infants born at high altitude or tis by the age of 40 years, and two thirds die by the
prematurely. age of 60 years.11,36
A patient with patent ductus arteriosus and a mod- Surgical ligation of patent ductus arteriosus, gen-
erate or large shunt has bounding peripheral arterial erally accomplished without cardiopulmonary bypass,
pulses, a widened pulse pressure, and a hyperdynamic has an associated mortality of less than 0.5 percent.
left ventricular impulse. The first heart sound is nor- However, in patients with ductal aneurysmal dilata-
mal. A continuous machinery murmur, audible in tion or calcification, resection with cardiopulmonary
the second left anterior intercostal space, begins short- bypass may be required.40,42 Because of the risk of en-
ly after the first heart sound, peaks in intensity at or darteritis associated with unrepaired patent ductus
immediately after the second heart sound (thereby arteriosus (estimated at 0.45 percent annually after
obscuring it), and declines in intensity during dias- the second decade of life)36 and the low risk associ-
tole. With a large shunt, mid-diastolic and systolic ated with ligation, we recommend that even a small
murmurs (from increased flow through the mitral patent ductus arteriosus be ligated surgically or oc-
Vol ume 342 Numb e r 4 259
The New England Journal of Medicine
Downloaded from nejm.org at UNIVERSIDAD NACIONAL AUTONOMA DE MEXICO on May 21, 2017. For personal use only. No other uses without permission.
Copyright 2000 Massachusetts Medical Society. All rights reserved.
The Ne w E n g l a nd Jo u r n a l o f Me d ic i ne
cluded with a percutaneously placed closure device. tients with symptomatic aortic stenosis should un-
Once severe pulmonary vascular obstructive disease dergo valve replacement.
develops, surgical ligation or percutaneous closure is
contraindicated.40,43 Pulmonary Stenosis
Pulmonary stenosis constitutes 10 to 12 percent
Aortic Stenosis of the cases of congenital heart disease in adults.
The most common pathological finding in patients Obstruction of right ventricular outflow is valvular
with symptomatic aortic stenosis who are younger in 90 percent of patients, and in the remainder it is
than 65 years of age is a bicuspid aortic valve, which supravalvular or subvalvular. Supravalvular pulmonary
is found in 2 to 3 percent of the population.44 It is stenosis results from the narrowing of the pulmonary
four times as common in men and boys as in women trunk, its bifurcation, or its peripheral branches; it
and girls. Twenty percent of patients with bicuspid often coexists with other congenital cardiac abnormal-
aortic valve have an associated cardiovascular abnor- ities (valvular pulmonary stenosis, atrial septal defect,
mality,45 such as patent ductus arteriosus or aortic ventricular septal defect, patent ductus arteriosus, or
coarctation. In patients with bicuspid aortic valve, the tetralogy of Fallot). It is a common feature of Wil-
bicuspid valve has a single fused commissure and an liams syndrome,47 which is characterized by infantile
eccentrically oriented orifice. Although the deformed hypercalcemia, elfin facies, and mental retardation, in
valve is not stenotic at birth, it is subjected to abnor- addition to supravalvular pulmonary stenosis. Sub-
mal hemodynamic stress, which may lead to thicken- valvular pulmonary stenosis, which is caused by the
ing and calcification of the leaflets, with resultant narrowing of the right ventricular infundibulum or
immobility. In many patients, there is a coexisting ab- subinfundibulum, usually occurs in association with
normality of the medial layer of the aorta above the a ventricular septal defect.
valve, which predisposes patients to have dilatation Valvular pulmonary stenosis typically is an isolated
of the aortic root. The area of the aortic orifice in a abnormality, but it may occur in association with ven-
normal adult is 3.0 to 4.0 cm 2. Aortic stenosis does tricular septal defect or lead to secondary hypertroph-
not become hemodynamically important unless the ic subpulmonary stenosis. The valve leaflets usually are
valve area is reduced to approximately 1.0 cm2. thin and pliant; all three valve cusps are present; and
In patients with severe aortic stenosis, the carotid the commissures are fused, so that during ventricu-
upstroke is usually delayed and diminished, but it may lar systole the valve is dome-shaped with a small cen-
be normal in elderly patients with noncompliant ca- tral orifice. Among patients with valvular stenosis, 10
rotid arteries. The aortic component of the second to 15 percent have dysplastic leaflets, which are thick-
heart sound is diminished or inaudible, and a fourth ened, immobile, and composed of myxomatous tissue.
heart sound is present. A harsh systolic crescendo About two thirds of patients with Noonans syndrome
decrescendo murmur is audible over the aortic area have pulmonary stenosis due to valve dysplasia.48
and often radiates to the neck. As the aortic steno- The area of the pulmonary-valve orifice in a normal
sis worsens, the murmur peaks progressively later in adult is about 2.0 cm 2 per square meter of body-sur-
systole. face area, and there is no systolic pressure gradient
Left ventricular hypertrophy results from gradual- across the valve. When the valve becomes stenotic,
ly worsening aortic stenosis and is usually evident on the right ventricular systolic pressure increases and a
electrocardiography. Unless the left ventricle dilates, systolic pressure gradient is observed between the
the chest x-ray film demonstrates a normal cardiotho- right ventricle and pulmonary artery. Pulmonary ste-
racic silhouette. In most patients, transthoracic echo- nosis is considered mild if the valve area is larger than
cardiography with Doppler flow permits an accurate 1.0 cm 2 per square meter, the transvalvular gradient
assessment of the severity of the stenosis and of left is less than 50 mm Hg, or the peak right ventricular
ventricular systolic function. Cardiac catheterization systolic pressure is less than 75 mm Hg. Pulmonary
is performed to determine the severity of aortic ste- stenosis is considered moderate if the valve area is 0.5
nosis in cases in which it cannot be assessed nonin- to 1.0 cm 2 per square meter, the transvalvular gradi-
vasively and to determine whether concomitant cor- ent is 50 to 80 mm Hg, or the right ventricular sys-
onary artery disease is present. tolic pressure is 75 to 100 mm Hg. Severe pulmonary
The classic symptoms of aortic stenosis are angina stenosis is characterized by a valve area of less than
pectoris, syncope or near-syncope, and heart failure. 0.5 cm 2 per square meter, a transvalvular gradient of
Adults with aortic stenosis who are asymptomatic more than 80 mm Hg, or a right ventricular systolic
have a normal life expectancy; they should receive pressure of more than 100 mm Hg.49,50
antibiotic prophylaxis against infective endocarditis. In patients with moderate or severe pulmonary
Once symptoms appear, survival is limited: the me- stenosis, a right ventricular impulse may be palpated
dian survival is only five years after angina develops, at the left sternal border, and there may be a thrill at
three years after syncope occurs, and two years after the second left intercostal space. The first heart sound
symptoms of heart failure appear.46 Therefore, pa- is normal, and the second heart sound is widely split
260 Ja nu ar y 2 7 , 2 0 0 0
The New England Journal of Medicine
Downloaded from nejm.org at UNIVERSIDAD NACIONAL AUTONOMA DE MEXICO on May 21, 2017. For personal use only. No other uses without permission.
Copyright 2000 Massachusetts Medical Society. All rights reserved.
MED ICA L PROGR ES S
but moves normally with respiration; its pulmonary in intervention offers no advantage. Balloon valvulo-
component is soft and delayed. A harsh crescendo plasty, the procedure of choice, is usually successful,
decrescendo systolic murmur that increases in inten- provided the valve is mobile and pliant; its long-term
sity with inspiration is audible along the left sternal results are excellent.51-55 The secondary hypertrophic
border. If the valve is pliable, an ejection click often subpulmonary stenosis that may occur with valvular
precedes the murmur; typically, the click softens or stenosis usually regresses after successful interven-
disappears with inspiration. As the stenosis becomes tion.52,54 Valve replacement is required if the leaflets
more severe, the systolic murmur peaks later in sys- are dysplastic or calcified or if marked regurgitation
tole and the ejection click moves closer to the first is present.
heart sound, eventually becoming virtually superim-
posed on it. Aortic Coarctation
In cases of moderate or severe pulmonary steno- Coarctation of the aorta typically consists of a dis-
sis, the electrocardiogram shows right-axis deviation crete, diaphragm-like ridge extending into the aortic
and right ventricular hypertrophy. Post-stenotic dil- lumen just distal to the left subclavian artery at the
atation of the main pulmonary artery and dimin- site of the aortic ductal attachment (the ligamentum
ished pulmonary vascular markings are evident on arteriosum) (Fig. 4). This condition results in hyper-
radiography. The cardiac silhouette is usually normal tension in the arms. Less commonly, the coarctation
in size. An enlarged cardiac silhouette may be seen is immediately proximal to the left subclavian artery,
if the patient has right ventricular failure or tricuspid in which case a difference in arterial pressure is not-
regurgitation. On echocardiography, right ventricular ed between the arms. Extensive collateral arterial cir-
hypertrophy and paradoxical septal motion during culation to the distal body through the internal tho-
systole are evident. The site of obstruction can be vis- racic, intercostal, subclavian, and scapular arteries
ualized in most patients. With the use of Doppler frequently develops in patients with aortic coarcta-
flow studies, the severity of stenosis can usually be tion. The condition, which is two to five times as fre-
assessed, so that catheterization and angiography are
unnecessary.
The presence or absence of symptoms, their sever-
ity, and the prognosis are influenced by the severity
of stenosis, the right ventricular systolic function, and Coarctation
the competence of the tricuspid valve.50 Adults with Axillary artery
valvular pulmonary stenosis are often asymptomatic;
in such patients the condition is identified by auscul-
tation of a loud systolic murmur. When the stenosis is
severe, dyspnea on exertion or fatigability may occur;
less often, patients may have retrosternal chest pain or
syncope with exertion. Eventually, right ventricular
failure may develop, with resultant peripheral edema
and abdominal swelling. Finally, if the foramen ovale
is patent, shunting of blood from the right to the left
atrium may occur, causing cyanosis and clubbing.
Adults with mild valvular pulmonary stenosis are
usually asymptomatic; in such patients the condition
does not require correction. Survival among such pa-
tients is excellent, with 94 percent still alive 20 years
after diagnosis.49 Patients with mild valvular stenosis Ascending-
who are undergoing elective dental or surgical proce- aorta
dures should receive antibiotic prophylaxis against in-
fective endocarditis. In contrast, patients with severe Intercostal-
stenosis should have the stenosis relieved, since only Ligamentum- Descending- arteries
40 percent of such patients do not require any inter- arteriosum aorta Internal-
vention by 10 years after diagnosis.49 Patients with Pulmonary- thoracic-
moderate pulmonary stenosis have an excellent prog- artery artery
nosis with either medical or interventional therapy.49
Interventional therapy is usually recommended, since
Figure 4. Coarctation of the Aorta.
most patients with moderate pulmonary stenosis even-
Coarctation causes severe obstruction of blood flow in the de-
tually have symptoms requiring such therapy. Relief of scending thoracic aorta. The descending aorta and its branches
valvular stenosis can be accomplished easily and safely are perfused by collateral channels from the axillary and inter-
with percutaneous balloon valvuloplasty, and a delay nal thoracic arteries through the intercostal arteries (arrows).
Vol ume 342 Numb e r 4 261
The New England Journal of Medicine
Downloaded from nejm.org at UNIVERSIDAD NACIONAL AUTONOMA DE MEXICO on May 21, 2017. For personal use only. No other uses without permission.
Copyright 2000 Massachusetts Medical Society. All rights reserved.
The Ne w E n g l a nd Jo u r n a l o f Me d ic i ne
quent in men and boys as in women and girls, may over the age of 40 years who have uncorrected aor-
occur in conjunction with gonadal dysgenesis (e.g., tic coarctation have symptoms of heart failure. Three
Turners syndrome), bicuspid aortic valve, ventricu- fourths die by the age of 50, and 90 percent by the
lar septal defect, patent ductus arteriosus, mitral ste- age of 60.11,57
nosis or regurgitation, or aneurysms of the circle of Surgical repair should be considered for patients
Willis.11,56 with a transcoarctation pressure gradient of more than
On physical examination, the systolic arterial pres- 30 mm Hg. Although balloon dilatation is a thera-
sure is higher in the arms than in the legs, but the di- peutic alternative, the procedure is associated with a
astolic pressures are similar; therefore, a widened pulse higher incidence of subsequent aortic aneurysm and
pressure is present in the arms. The femoral arterial recurrent coarctation than surgical repair.58 Postop-
pulses are weak and delayed. A systolic thrill may be erative complications include residual or recurrent hy-
palpable in the suprasternal notch, and left ventricular pertension, recurrent coarctation, and the possible
enlargement may be noted. A systolic ejection click sequelae of a bicuspid aortic valve.59 The incidence
(due to a bicuspid aortic valve) is frequently present, of persistent or recurrent hypertension, as well as the
and the second heart sound is accentuated. A harsh survival rate, is influenced by the patients age at the
systolic ejection murmur may be identified along the time of surgery. Among patients who undergo sur-
left sternal border and in the back, particularly over gery during childhood, 90 percent are normotensive
the coarctation. A systolic murmur, caused by flow 5 years later, 50 percent are normotensive 20 years lat-
through collateral vessels, may be heard in the back. er, and 25 percent are normotensive 25 years later.11
In about 30 percent of patients with aortic coarcta- In contrast, among those who undergo surgery after
tion, a systolic murmur indicating an associated bi- the age of 40 years, half have persistent hypertension,
cuspid aortic valve is audible at the base. and many of those with a normal resting blood pres-
The electrocardiogram usually shows left ventric- sure after successful repair have a hypertensive response
ular hypertrophy. On the chest radiograph, increased to exercise.
collateral flow through the intercostal arteries causes Similarly, survival after repair of aortic coarctation
notching of the posterior third of the third through is also influenced by the age of the patient at the time
eighth ribs; such notching is usually symmetric. of surgery. After surgical repair during childhood,
Notching is not seen in the anterior ribs, since the 89 percent of patients are alive 15 years later and 83
anterior intercostal arteries are not located in costal percent are alive 25 years later. When repair of co-
grooves. The coarctation may be visible as an inden- arctation is performed when the patient is between
tation of the aorta, and one may see prestenotic and the ages of 20 and 40 years, the 25-year survival is 75
poststenotic dilatation of the aorta, producing the percent. When repair is performed in patients more
reversed E or 3 sign. The coarctation may be than 40 years old, the 15-year survival is only 50
visualized echocardiographically, and Doppler exam- percent.11,60
ination makes possible an estimate of the transco-
REFERENCES
arctation pressure gradient. Computed tomography,
magnetic resonance imaging, and contrast aortogra- 1. Moodie DS. Adult congenital heart disease. Curr Opin Cardiol 1994;
9:137-42.
phy provide precise anatomical information regard- 2. Feldt RH, Avasthey P, Yoshimasu F, Kurland LT, Titus JL. Incidence of
ing the location and length of the coarctation; in ad- congenital heart disease in children born to residents of Olmsted County,
dition, aortography permits the visualization of the Minnesota, 1950-1969. Mayo Clin Proc 1971;46:794-9.
3. Campbell M. Natural history of atrial septal defect. Br Heart J 1970;
collateral circulation. 32:820-6.
Most adults with aortic coarctation are asympto- 4. Leachman RD, Cokkinos DV, Cooley DA. Association of ostium secun-
matic. The diagnosis is made during routine physical dum atrial septal defects with mitral valve prolapse. Am J Cardiol 1976;38:
167-9.
examination, when systemic arterial hypertension is 5. Van Praagh S, Carrera ME, Sanders SP, Mayer JE, Van Praagh R. Sinus
observed in the arms, with diminished or absent fem- venosus defects: unroofing of the right pulmonary veins anatomic and
echocardiographic findings and surgical treatment. Am Heart J 1994;128:
oral arterial pulses. When symptoms are present, they 365-79.
are usually those of hypertension: headache, epistax- 6. Holt M, Oram S. Familial heart disease with skeletal malformations. Br
is, dizziness, and palpitations. Occasionally, dimin- Heart J 1960;22:236-42.
7. Basson CT, Cowley GS, Solomon SD, et al. The clinical and genetic
ished blood flow to the legs causes claudication. Pa- spectrum of the HoltOram syndrome (hearthand syndrome). N Engl
tients sometimes seek medical attention because they J Med 1994;330:885-91. [Erratum, N Engl J Med 1994;330:1627.]
have symptoms of heart failure or aortic dissection. 8. Lynch HT, Bachenberg K, Harris RE, Becker W. Hereditary atrial septal
defect: update of a large kindred. Am J Dis Child 1978;132:600-4.
Women with coarctation are at particularly high risk 9. Pease WE, Nordenberg A, Ladda RL. Familial atrial septal defect with
for aortic dissection during pregnancy. prolonged atrioventricular conduction. Circulation 1976;53:759-62.
10. OToole JD, Reddy PS, Curtiss EI, Shaver JA. The mechanism of split-
Complications of aortic coarctation include hyper- ting of the second heart sound in atrial septal defect. Circulation 1977;56:
tension, left ventricular failure, aortic dissection, pre- 1047-53.
mature coronary artery disease, infective endocarditis, 11. Perloff JK. Survival patterns without cardiac surgery or interventional
catheterization: a narrowing base. In: Perloff JK, Childs JS, eds. Congenital
and cerebrovascular accidents (due to the rupture of heart disease in adults. 2nd ed. Philadelphia: W.B. Saunders, 1998:15-53.
an intracerebral aneurysm). Two thirds of patients 12. Shub C, Dimopoulos IN, Seward JB, et al. Sensitivity of two-dimen-
262 Ja nu ar y 2 7 , 2 0 0 0
The New England Journal of Medicine
Downloaded from nejm.org at UNIVERSIDAD NACIONAL AUTONOMA DE MEXICO on May 21, 2017. For personal use only. No other uses without permission.
Copyright 2000 Massachusetts Medical Society. All rights reserved.
MED ICA L PROGR ES S
sional echocardiography in the direct visualization of atrial septal defect 37. Ohtsuka S, Kakihana M, Ishikawa T, et al. Aneurysm of patent ductus
utilizing the subcostal approach: experience with 154 patients. J Am Coll arteriosus in an adult case: findings of cardiac catheterization, angiography,
Cardiol 1983;2:127-35. and pathology. Clin Cardiol 1987;10:537-40.
13. Adams CW. A reappraisal of life expectancy with atrial shunts of the 38. Lund JT, Jensen MB, Hjelms E. Aneurysm of the ductus arteriosus:
secundum type. Dis Chest 1965;48:357-75. a review of the literature and the surgical implications. Eur J Cardiothorac
14. Craig RJ, Selzer A. Natural history and prognosis of atrial septal de- Surg 1991;5:566-70.
fect. Circulation 1968;37:805-15. 39. Kelly DT. Patent ductus arteriosus in adults. Cardiovasc Clin 1979;10:
15. Markman P, Howitt G, Wade EG. Atrial septal defect in the middle- 321-6.
aged and elderly. QJM 1965;34:409-26. 40. Fisher RG, Moodie DS, Sterba R, Gill CC. Patent ductus arteriosus in
16. Mattila S, Merikallio E, Tala P. ASD in patients over 40 years of age. adults long-term follow-up: nonsurgical versus surgical treatment. J Am
Scand J Thorac Cardiovasc Surg 1979;13:21-4. Coll Cardiol 1986;8:280-4.
17. Perloff JK. Ostium secundum atrial septal defect survival for 87 and 41. Espino-Vela J, Cardenas N, Cruz R. Patent ductus arteriosus: with spe-
94 years. Am J Cardiol 1984;53:388-9. cial reference to patients with pulmonary hypertension. Circulation 1968;
18. Murphy JG, Gersh BJ, McGoon MD, et al. Long-term outcome after 38:Suppl I:I-45I-60.
surgical repair of isolated atrial septal defect: follow-up at 27 to 32 years. 42. Bell-Thomson J, Jewell E, Ellis FH Jr, Schwaber JR. Surgical technique
N Engl J Med 1990;323:1645-50. in the management of patent ductus arteriosus in the elderly patient. Ann
19. Konstantinides S, Geibel A, Olschewski M, et al. A comparison of sur- Thorac Surg 1980;30:80-3.
gical and medical therapy for atrial septal defect in adults. N Engl J Med 43. John S, Muralidharan S, Jairaj PS, et al. The adult ductus: review of
1995;333:469-73. surgical experience with 131 patients. J Thorac Cardiovasc Surg 1981;82:
20. Gatzoulis MA, Freeman MA, Siu SC, Webb GD, Harris L. Atrial ar- 314-9.
rhythmia after surgical closure of atrial septal defects in adults. N Engl J 44. Subramanian R, Olson LJ, Edwards WD. Surgical pathology of pure
Med 1999;340:839-46. aortic stenosis: a study of 374 cases. Mayo Clin Proc 1984;59;683-90.
21. Steele PM, Fuster V, Cohen M, Ritter DG, McGoon DC. Isolated 45. Friedman WF. Aortic stenosis. In: Emmanouilides GC, Riemen-
atrial septal defect with pulmonary vascular obstructive disease long- schneider TA, Allen HD, Gutgesell HP, eds. Moss and Adams heart disease
term follow-up and prediction of outcome after surgical correction. Circu- in infants, children, and adolescents. Baltimore: Williams & Wilkins, 1995:
lation 1987;76:1037-42. 1087-111.
22. Rickers C, Hamm C, Stern H, et al. Percutaneous closure of secun- 46. Carabello BA, Crawford FA Jr. Valvular heart disease. N Engl J Med
dum atrial septal defect with a new self centering device (angel wings). 1997;337:32-41. [Erratum, N Engl J Med 1997;337:507.]
Heart 1998;80:517-21. 47. Zalzstein E, Moes CA, Musewe NN, Freedom RM. Spectrum of car-
23. Lambert V, Losay J, Piot J-D, et al. Complications tardives aprs fer- diovascular anomalies in Williams-Beuren syndrome. Pediatr Cardiol 1991;
meture percutane des communications interauriculaires par prothse bou- 12:219-23.
tonne de Sideris. Arch Mal Coeur Vaiss 1997;90:245-51. 48. Pearl W. Cardiovascular anomalies in Noonans syndrome. Chest 1977;
24. Dajani AS, Taubert KA, Wilson W, et al. Prevention of bacterial en- 71:677-9.
docarditis: recommendations by the American Heart Association. JAMA 49. Hayes CJ, Gersony WM, Driscoll DJ, et al. Second natural history
1997;277:1794-801. study of congenital heart defects: results of treatment of patients with pul-
25. Weidman WH, DuShane JW, Ellison RC. Clinical course in adults monary valvar stenosis. Circulation 1993;87:Suppl I:I-28I-37.
with ventricular septal defect. Circulation 1977;56:Suppl I:I-78I-79. 50. Kaplan S, Perloff JK. Exercise and athletics before and after cardiac
26. Graham TP Jr, Gutgesell HP. Ventricular septal defects. In: Emman- surgery or interventional catheterization. In: Perloff JK, Childs JC, eds.
ouilides GC, Riemenschneider TA, Allen HD, Gutgesell HP, eds. Moss Congenital heart disease in adults. 2nd ed. Philadelphia: W.B. Saunders,
and Adams heart disease in infants, children, and adolescents. Baltimore: 1998:189-98.
Williams & Wilkins, 1995:724-46. 51. Sadr-Ameli MA, Sheikholeslami F, Firoozi I, Azarnik H. Late results
27. Pieroni DR, Nishimura RA, Bierman FZ, et al. Second natural history of balloon pulmonary valvuloplasty in adults. Am J Cardiol 1998;82:398-
study of congenital heart defects: ventricular septal defect: echocardiography. 400.
Circulation 1993;87:Suppl I:I-80I-88. 52. Teupe CH, Burger W, Schrader R, Zeiher AM. Late (five to nine
28. Valdes-Cruz LM, Cayre RO. Ventricular septal defects. In: Valdes- years) follow-up after balloon dilation of valvular pulmonary stenosis in
Cruz LM, Cayre RO, eds. Echocardiographic diagnosis of congenital heart adults. Am J Cardiol 1997;80:240-2.
disease: an embryologic and anatomic approach. Philadelphia: Lippincott- 53. Chen C-R, Cheng TO, Huang T, et al. Percutaneous balloon valvulo-
Raven, 1999:199-213. plasty for pulmonic stenosis in adolescents and adults. N Engl J Med 1996;
29. Boehrer JD, Lange RA, Willard JE, Grayburn PA, Hillis LD. Advan- 335:21-5.
tages and limitations of methods to detect, localize, and quantitate intra- 54. Fawzy ME, Galal O, Dunn B, Shaikh A, Sriram R, Duran CM. Re-
cardiac left-to-right shunting. Am Heart J 1992;124:448-55. gression of infundibular pulmonary stenosis after successful balloon pul-
30. Kidd L, Driscoll DJ, Gersony WM, et al. Second natural history study monary valvuloplasty in adults. Cathet Cardiovasc Diagn 1990;21:77-81.
of congenital heart defects: results of treatment of patients with ventricular 55. Kaul UA, Singh B, Tyagi S, Bhargava M, Arora R, Khalilullah M.
septal defects. Circulation 1993;87:Suppl I:I-38I-51. Long-term results after balloon pulmonary valvuloplasty in adults. Am
31. Neumayer U, Stone S, Somerville J. Small ventricular septal defects in Heart J 1993;126:1152-5.
adults. Eur Heart J 1998;19:1573-82. 56. Mazzanti L, Prandstraller D, Tassinari D, et al. Heart disease in Tur-
32. Gersony WM, Hayes CJ, Driscoll DJ, et al. Bacterial endocarditis in ners syndrome. Helv Paediatr Acta 1988;43:25-31.
patients with aortic stenosis, pulmonary stenosis, or ventricular septal de- 57. Campbell M. Natural history of coarctation of the aorta. Br Heart J
fect. Circulation 1993;87:Suppl I:I-121I-126. 1970;32:633-40.
33. Takenaka K, Sakamoto T, Shiota T, Amano W, Igarashi T, Sugimoto 58. Shaddy RE, Boucek MM, Sturtevant JE, et al. Comparison of angio-
T. Diagnosis of patent ductus arteriosus in adults by biplane transesopha- plasty and surgery for unoperated coarctation of the aorta. Circulation
geal color Doppler flow mapping. Am J Cardiol 1991;68:691-3. 1993;87:793-9.
34. Coggin CJ, Parker KR, Keith JD. Natural history of isolated patent 59. Cohen M, Fuster V, Steele PM, Driscoll D, McGoon DC. Coarctation
ductus arteriosus and the effect of surgical correction: twenty years expe- of the aorta: long-term follow-up and prediction of outcome after surgical
rience at the Hospital for Sick Children, Toronto. CMAJ 1970;102:718-20. correction. Circulation 1989;80:840-5.
35. Campbell M. Patent ductus arteriosus: some notes on prognosis and 60. Coarctation of the aorta and interrupted aortic arch. In: Kirklin JW,
on pulmonary hypertension. Br Heart J 1955;17:511-33. Barratt-Boyes BG. Cardiac surgery: morphology, diagnostic criteria, natu-
36. Idem. Natural history of persistent ductus arteriosus. Br Heart J 1968; ral history, techniques, results, and indications. 2nd ed. Vol. 2. New York:
30:4-13. Churchill Livingstone, 1993:1263-325.
Vol ume 342 Numb e r 4 263
The New England Journal of Medicine
Downloaded from nejm.org at UNIVERSIDAD NACIONAL AUTONOMA DE MEXICO on May 21, 2017. For personal use only. No other uses without permission.
Copyright 2000 Massachusetts Medical Society. All rights reserved.
You might also like
- Heart Failure (Muir)Document5 pagesHeart Failure (Muir)Ganashiam NadarasaNo ratings yet
- PJB Pada Dewasa AASDocument54 pagesPJB Pada Dewasa AAS1e23e2ewNo ratings yet
- 41 HLHS Ma AaDocument14 pages41 HLHS Ma AaVictor PazNo ratings yet
- Physiology Congenital: of DiseaseDocument5 pagesPhysiology Congenital: of DiseaseFirah Triple'sNo ratings yet
- Lapkas Dian and DamboDocument34 pagesLapkas Dian and DamboDian Primadia PutriNo ratings yet
- Atrial Septal Defect (ASD) Dan Ventricular Septal Defect (VSD)Document10 pagesAtrial Septal Defect (ASD) Dan Ventricular Septal Defect (VSD)Anis Syafa'atul HusnaNo ratings yet
- (Lec - Oleson) CHDDocument89 pages(Lec - Oleson) CHDMathew JosephNo ratings yet
- Cvs2-k1 (JP-PJB Pada Dewasa)Document81 pagesCvs2-k1 (JP-PJB Pada Dewasa)32 sebastian Josia N.No ratings yet
- Atrial Septal DefectDocument8 pagesAtrial Septal DefectWidelmark FarrelNo ratings yet
- Minggu 2 LP ASDDocument15 pagesMinggu 2 LP ASDMuhammad PanduNo ratings yet
- Atrial Septal Defect: Go ToDocument10 pagesAtrial Septal Defect: Go ToanisNo ratings yet
- Ciano Tico IDocument14 pagesCiano Tico ILilik FitrianaNo ratings yet
- Congenital Heart DiseaseDocument93 pagesCongenital Heart DiseaseManjunatha HR100% (1)
- ASD VSD Tugas Dr. IstiDocument10 pagesASD VSD Tugas Dr. IstiAnis Syafa'atul HusnaNo ratings yet
- The Surgical Management of Ebstein Anomaly: ReviewDocument8 pagesThe Surgical Management of Ebstein Anomaly: ReviewSanjukta BoseNo ratings yet
- AnnCardAnaesth10119-300904 082130 PDFDocument8 pagesAnnCardAnaesth10119-300904 082130 PDFFirah Triple'sNo ratings yet
- A Case of Ruptured Coronary Sinus of ValsalvaDocument19 pagesA Case of Ruptured Coronary Sinus of Valsalvajb_blasurca100% (1)
- RHF and Congenital Heart DiseaseDocument4 pagesRHF and Congenital Heart DiseasePedro AugustoNo ratings yet
- By: Jacqueline I. Esmundo, R.N.MNDocument32 pagesBy: Jacqueline I. Esmundo, R.N.MNdomlhynNo ratings yet
- Congenital Heart Disease EtiologyDocument8 pagesCongenital Heart Disease EtiologykudzaimuregidubeNo ratings yet
- Pathophysiology of Congenital Heart Diseases PDFDocument8 pagesPathophysiology of Congenital Heart Diseases PDFdramitjainNo ratings yet
- Pathophysiology of Congenital Heart Diseases PDFDocument8 pagesPathophysiology of Congenital Heart Diseases PDFkishanNo ratings yet
- Congenital Heart Disease For The Adult Cardiologist: Transposition of The Great ArteriesDocument12 pagesCongenital Heart Disease For The Adult Cardiologist: Transposition of The Great ArteriesSaid hanNo ratings yet
- Congenital Heart Disease For The Adult CardiologistDocument12 pagesCongenital Heart Disease For The Adult CardiologistAndreea SocolNo ratings yet
- FinalDocument56 pagesFinalvamshidhNo ratings yet
- Atrial Septal DefectDocument5 pagesAtrial Septal Defectalfiansyah syrfdnNo ratings yet
- Congenital Heart Disease - Part IDocument95 pagesCongenital Heart Disease - Part IMeraol HusseinNo ratings yet
- Acyanotic Congenital Heart DiseaseDocument7 pagesAcyanotic Congenital Heart DiseaseSam Raj100% (1)
- Ventricular Septal DefectDocument11 pagesVentricular Septal DefectFajar YuniftiadiNo ratings yet
- Ventricular Septal Defects in AdultsDocument13 pagesVentricular Septal Defects in AdultsМихаил НеболеевNo ratings yet
- Congenital Heart DiseaseDocument10 pagesCongenital Heart DiseaseIca JustitiaNo ratings yet
- Fishbein 2019Document8 pagesFishbein 2019hawk.man8No ratings yet
- Ruptured Sinus ValsalvaDocument16 pagesRuptured Sinus ValsalvaKhandar YosuaNo ratings yet
- Pulmonary Embolism: Here Is Where Your Presentation BeginsDocument39 pagesPulmonary Embolism: Here Is Where Your Presentation BeginsAsmaa ahmedNo ratings yet
- Pathophysiology and Natural History of Atrial Septal DefectDocument10 pagesPathophysiology and Natural History of Atrial Septal DefectrosaNo ratings yet
- 45 Tricuspid AtresiaDocument7 pages45 Tricuspid AtresiaVictor PazNo ratings yet
- Current Management of Ebstein's Anomaly in The AdultDocument9 pagesCurrent Management of Ebstein's Anomaly in The AdultRJMNo ratings yet
- 52 Vascular FistulaeDocument6 pages52 Vascular FistulaeVictor PazNo ratings yet
- Aortic Regurgitation: Clinical PracticeDocument8 pagesAortic Regurgitation: Clinical PracticeChintya Fidelia MontangNo ratings yet
- Conginital Heart DiseaseDocument19 pagesConginital Heart DiseaseSanthosh.S.UNo ratings yet
- Atrial Septal Defects: Presented by Dr. Maysa Abdul Haq Directed by Dr. Ali Halabi Jordan Hospital 11-9-2005Document36 pagesAtrial Septal Defects: Presented by Dr. Maysa Abdul Haq Directed by Dr. Ali Halabi Jordan Hospital 11-9-2005Joe JosephNo ratings yet
- ASD Atrial Septal Defect PDFDocument9 pagesASD Atrial Septal Defect PDFAco AjjahNo ratings yet
- Case Report - An Uncommon Association of Ebstein's Anomaly and Rheumatic Mitral StenosisDocument4 pagesCase Report - An Uncommon Association of Ebstein's Anomaly and Rheumatic Mitral StenosisRJMNo ratings yet
- Aortopulmonary Window in InfantsDocument3 pagesAortopulmonary Window in Infantsonlyjust4meNo ratings yet
- Tetralogy of FallotDocument37 pagesTetralogy of Fallottintinlovessu100% (1)
- Ebsteins Anomaly-An OverviewDocument36 pagesEbsteins Anomaly-An OverviewRJMNo ratings yet
- Usm UvodDocument12 pagesUsm UvodMicija CucuNo ratings yet
- Atrial Septal DefectDocument3 pagesAtrial Septal Defectktin17No ratings yet
- Anatomical and Pathophysiological Classification of Congenital Heart DiseaseDocument16 pagesAnatomical and Pathophysiological Classification of Congenital Heart DiseasePietro PensoNo ratings yet
- Cardiovascular DysfunctionDocument15 pagesCardiovascular DysfunctionJhasseryne Orias SanchezNo ratings yet
- Cctga PcicsDocument9 pagesCctga PcicsAdrian KhomanNo ratings yet
- Ebstein Anomaly in The Adult PatientDocument11 pagesEbstein Anomaly in The Adult PatientRJMNo ratings yet
- CVS - Session 3 - CHDDocument3 pagesCVS - Session 3 - CHDkotecha.rheaNo ratings yet
- Case Study: Double Outlet Right VentricleDocument6 pagesCase Study: Double Outlet Right VentriclejisooNo ratings yet
- Anomalous Origin of The Coronary Artery From The Pulmonary Artery in Children and Adults - A Pictorial Review of Cardiac Imaging FindingsDocument10 pagesAnomalous Origin of The Coronary Artery From The Pulmonary Artery in Children and Adults - A Pictorial Review of Cardiac Imaging FindingsChici FarlinaNo ratings yet
- Cayanotic Heart DiseaseDocument28 pagesCayanotic Heart DiseasesivanathanNo ratings yet
- Lapkas Anak - Qarina Dian EditedDocument28 pagesLapkas Anak - Qarina Dian EditedDian Primadia PutriNo ratings yet
- Atrial Septal Defect: J F. K, T G, D C. FDocument14 pagesAtrial Septal Defect: J F. K, T G, D C. FVictor PazNo ratings yet
- Atrial Septal Defect - A ReviewDocument5 pagesAtrial Septal Defect - A ReviewInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Irritable Bowel SyndromeDocument24 pagesIrritable Bowel SyndromeTony Gomez Luna LeyvaNo ratings yet
- Etiquette-Based Medicine: PerspectiveDocument2 pagesEtiquette-Based Medicine: PerspectiveTony Gomez Luna LeyvaNo ratings yet
- Bacterial Skin and Soft Tissue Infections Pathogenesis, EpidemiologyDocument11 pagesBacterial Skin and Soft Tissue Infections Pathogenesis, EpidemiologyTony Gomez Luna Leyva0% (1)
- 2013 Cardiac Pacing and CRT 2013 ESC GuidelineDocument49 pages2013 Cardiac Pacing and CRT 2013 ESC GuidelineTony Gomez Luna LeyvaNo ratings yet
- Abordaje Linfadenopatia PerifericaDocument13 pagesAbordaje Linfadenopatia PerifericaTony Gomez Luna LeyvaNo ratings yet
- The Metabolic Syndrome: Requiescat in Pace: Gerald M. ReavenDocument8 pagesThe Metabolic Syndrome: Requiescat in Pace: Gerald M. ReavenTony Gomez Luna LeyvaNo ratings yet
- 1.-Nature Review SGBDocument14 pages1.-Nature Review SGBTony Gomez Luna LeyvaNo ratings yet
- EtsDocument31 pagesEtsTony Gomez Luna LeyvaNo ratings yet
- Diagnostic Criteria For Acute Kidney Injury: Present and FutureDocument12 pagesDiagnostic Criteria For Acute Kidney Injury: Present and FutureTony Gomez Luna LeyvaNo ratings yet
- Chioma M. Okeoma (Eds.) - Chikungunya Virus - Advances in Biology, Pathogenesis, and Treatment-Springer International Publishing (2016)Document202 pagesChioma M. Okeoma (Eds.) - Chikungunya Virus - Advances in Biology, Pathogenesis, and Treatment-Springer International Publishing (2016)sayeed_opso100% (1)
- 14 - UveitisDocument4 pages14 - UveitisSpislgal PhilipNo ratings yet
- Therapeutic Apheresis Operator Competency Aug06Document43 pagesTherapeutic Apheresis Operator Competency Aug06Jose Gregorio Riobueno BolivarNo ratings yet
- Moduleiii:Summativeevaluation: Pheochromocytoma. (2020) - N Ational Library of M EdicineDocument1 pageModuleiii:Summativeevaluation: Pheochromocytoma. (2020) - N Ational Library of M EdicineKashley DangliNo ratings yet
- Immunization ScheduleDocument2 pagesImmunization ScheduleTracy100% (1)
- Lived Experiences of Individuals With Sexually Transmitted Infections (STIs) : An Input To Infectious Disease Awareness and PreventionDocument12 pagesLived Experiences of Individuals With Sexually Transmitted Infections (STIs) : An Input To Infectious Disease Awareness and PreventionPsychology and Education: A Multidisciplinary JournalNo ratings yet
- Basic First Aid NotesDocument9 pagesBasic First Aid NotesPaul SealyNo ratings yet
- Drugs Affecting Coagulation ObjectivesDocument14 pagesDrugs Affecting Coagulation ObjectiveslouradelNo ratings yet
- Lower GIT BleedingDocument42 pagesLower GIT BleedingRawan HamzaNo ratings yet
- Dengue Fever EngDocument17 pagesDengue Fever EngRia Tustina HendrayaniNo ratings yet
- DR Stuart Crisp DR Per Grinsted: Written byDocument8 pagesDR Stuart Crisp DR Per Grinsted: Written byRizky MarethaNo ratings yet
- Gonzaga Rlems - NCPDocument3 pagesGonzaga Rlems - NCPShaynne Wencille A. GONZAGANo ratings yet
- A Group of Homoeopathic Medicines For COVID 19: A Systematic Review of Clinical FeaturesDocument18 pagesA Group of Homoeopathic Medicines For COVID 19: A Systematic Review of Clinical FeaturesY.rajuNo ratings yet
- Astigmidwife Vlogs Online Tutorial: All Rights Are Reserved. No Part of This Publication May Be ReproducedDocument12 pagesAstigmidwife Vlogs Online Tutorial: All Rights Are Reserved. No Part of This Publication May Be ReproducedLynden BulanNo ratings yet
- Immuno Flow CytometryDocument8 pagesImmuno Flow CytometrySyed Hassan Raza NaqviNo ratings yet
- Current Concept Inguinal Hernia RepairDocument6 pagesCurrent Concept Inguinal Hernia RepairdewiswahyuNo ratings yet
- Kertas Kerja PinjamanDocument18 pagesKertas Kerja PinjamanWan AzmanNo ratings yet
- Waiver For RSPC Cliniquing 2018 p2Document1 pageWaiver For RSPC Cliniquing 2018 p2Rebecca MaderalNo ratings yet
- Fatigue After StrokeDocument6 pagesFatigue After StrokeAnugerah NasutionNo ratings yet
- What Are Early Indicators of Mesothelioma. Realizing The Early Signs ofDocument2 pagesWhat Are Early Indicators of Mesothelioma. Realizing The Early Signs ofthasyaNo ratings yet
- The Impact of Lifestyle Interventions On Type 2 Diabetes Management, A Comprehensive AnalysisDocument2 pagesThe Impact of Lifestyle Interventions On Type 2 Diabetes Management, A Comprehensive AnalysisJohn nyoikeNo ratings yet
- AlopeciaDocument9 pagesAlopeciaapi-502059670No ratings yet
- Oral PathologyDocument31 pagesOral PathologyAnonymous GyqTkXMwMNo ratings yet
- AFFERENT & EFFERENT SyndromeDocument12 pagesAFFERENT & EFFERENT SyndromeAli Sibra MulluziNo ratings yet
- 327-Differential Diagnosis of SEIZURE - SattawutDocument44 pages327-Differential Diagnosis of SEIZURE - Sattawutyusma wati99No ratings yet
- All India Ayush Post Graduate Entrance Test 2019 Question PaperDocument24 pagesAll India Ayush Post Graduate Entrance Test 2019 Question PaperSoumitra BoseNo ratings yet
- Rheum MCQDocument11 pagesRheum MCQMudassar Iqbal100% (2)
- Family Medicine Clerkship Logbook G3 FinalDocument76 pagesFamily Medicine Clerkship Logbook G3 FinalMohammed AlomarNo ratings yet
- Review: Arthritis in LeprosyDocument6 pagesReview: Arthritis in LeprosyadlestariNo ratings yet
- Cardiogenic SyokDocument51 pagesCardiogenic SyokRamadhyanNo ratings yet