Professional Documents
Culture Documents
Chemical Reaction Engineering - TKP4110 Exercise 1 - Fall 2013
Uploaded by
246theOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chemical Reaction Engineering - TKP4110 Exercise 1 - Fall 2013
Uploaded by
246theCopyright:
Available Formats
Chemical Reaction Engineering TKP4110
Exercise 1 Fall 2013
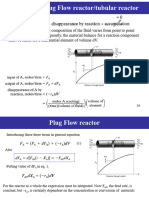
The irreversible liquid phase reaction:
A P
is to be carried out in a system with 2 continuous reactors. One continuously
stirred tank reactor (CSTR) with volume V CSTR and one plug flow reactor (PFR)
with volume VPFR. The volumetric feed rate is vo = 50 l/min and the initial
concentration is CAo. The reaction is adiabatic and non-elementary. The
following values have been determined experimentally:
XA
System 1: CSTR as reactor 1 and PFR as reactor 2.
System 2: PFR as reactor 1 and CSTR as reactor 2.
a) Consider the two systems shown above. Which of the two systems will
give the smallest reactor volume (total volume)?
b) Calculate the smallest reactor volume (total volume).
c) Does another solution exist (smallest possible reactor (total) volume) in
order to obtain a conversion of 70 % than the systems shown above?
d) What is the conversion that gives the same reactor volume for the CSTR
and the PFR?
e) The reaction is to be carried out in a CSTR with VCSTR=700 l. Use the
data above (CAo/-rA) =f(XA) as well as the reactor equation for CSTR to
make a figure with as a function of XA. What is the conversion(s) that
will be a result of this?
You might also like
- A B R KC K 0.5 Min: Tutorial 3Document2 pagesA B R KC K 0.5 Min: Tutorial 3shikharNo ratings yet
- CHE3044F, 2013: Reactor Design 1: TUTORIAL 6Document4 pagesCHE3044F, 2013: Reactor Design 1: TUTORIAL 6nmhatityeNo ratings yet
- Ech 4504 Summer 2017 HW 2Document1 pageEch 4504 Summer 2017 HW 2Ali MirNo ratings yet
- Tutorial 5drtuhDocument2 pagesTutorial 5drtuhFikrie MuhdNo ratings yet
- Tutorial QuestionsDocument8 pagesTutorial QuestionsMaame Efua Neizer100% (1)
- Assignment 2 Reactor Design Single ReactionDocument4 pagesAssignment 2 Reactor Design Single ReactionNitin MauryaNo ratings yet
- Assignment 4Document5 pagesAssignment 4Yi Hong LowNo ratings yet
- FullDocument33 pagesFullEja RotiKeju100% (2)
- Theory 1) The Reaction RateDocument3 pagesTheory 1) The Reaction RateDiyana Nabila Abdul WahidNo ratings yet
- NEC4101 - Session 2a - ReactorsDocument19 pagesNEC4101 - Session 2a - Reactorsclassfield tranNo ratings yet
- CHE 502 Tutorial 5Document3 pagesCHE 502 Tutorial 5Ibnu HamidNo ratings yet
- Design Equations F (X)Document26 pagesDesign Equations F (X)Raus FaadhilNo ratings yet
- Department of Chemical Engineering, Iit Delhi Reactor Sizing Problems Assignment-2Document2 pagesDepartment of Chemical Engineering, Iit Delhi Reactor Sizing Problems Assignment-2ShubhamGuptaNo ratings yet
- Example 2: Over What Range of Conversions Are The Plug-Flow Reactor and CSTR Volumes Identical?Document8 pagesExample 2: Over What Range of Conversions Are The Plug-Flow Reactor and CSTR Volumes Identical?Alejandra SanchezNo ratings yet
- Tut1 2016 QDocument5 pagesTut1 2016 QAbhishek SardaNo ratings yet
- Essy Questions On Non-Ideal ReactorsDocument9 pagesEssy Questions On Non-Ideal ReactorsRobinson ANo ratings yet
- Chemical Kinetics LectureDocument21 pagesChemical Kinetics LectureMohamed MegahedNo ratings yet
- P4E2: Kinetics of Homogeneous Reaction in Batch and Continuous Stirred-Tank Reactor at Two Different TemperatureDocument7 pagesP4E2: Kinetics of Homogeneous Reaction in Batch and Continuous Stirred-Tank Reactor at Two Different TemperaturejayaprinaNo ratings yet
- Taller 3 - 2016Document2 pagesTaller 3 - 2016LifeswolfsNo ratings yet
- Chemical Reactors - Problems of Reactor Association 47-60: (Exam Jan'09)Document6 pagesChemical Reactors - Problems of Reactor Association 47-60: (Exam Jan'09)Alfredo ZuñigaNo ratings yet
- CMC091Y1092 Chapter 2Document43 pagesCMC091Y1092 Chapter 2王暐翔No ratings yet
- CH 2Document22 pagesCH 2林哲璋No ratings yet
- Chemical Reactors: DC DT RDocument8 pagesChemical Reactors: DC DT ROsas Jessica UwoghirenNo ratings yet
- PFR Ilar TorrefielDocument16 pagesPFR Ilar TorrefielCastiel161No ratings yet
- Chapter 1 EDITED Student VersionDocument43 pagesChapter 1 EDITED Student VersionSyukri ShahNo ratings yet
- CHEE 321: Chemical Reaction Engineering: Module 3: Isothermal Reactor DesignDocument16 pagesCHEE 321: Chemical Reaction Engineering: Module 3: Isothermal Reactor DesignPranav NakhateNo ratings yet
- Plug Flow ReactorDocument16 pagesPlug Flow ReactorN Afiqah RazakNo ratings yet
- CENG 211 Reaction and Reactor Engineering (Fall, 1999)Document1 pageCENG 211 Reaction and Reactor Engineering (Fall, 1999)Ricardo VelozNo ratings yet
- L1 - Review Kinetics 1Document68 pagesL1 - Review Kinetics 1Christopher RamosNo ratings yet
- Exp - P2 - CSTRDocument6 pagesExp - P2 - CSTRSiddesh PatilNo ratings yet
- Cre 2020CDocument7 pagesCre 2020CRitul RajbangshiNo ratings yet
- Problem Set 5 T3 2013-2014Document2 pagesProblem Set 5 T3 2013-2014HolihuaChuaNo ratings yet
- Lecture 3 - Conversion and Reactor SizingDocument15 pagesLecture 3 - Conversion and Reactor Sizing88l8No ratings yet
- Temperature, °C: SolutionDocument18 pagesTemperature, °C: Solutionمحمد حلمي هاريريNo ratings yet
- CHEE 321: Chemical Reaction Engineering 2. Isothermal Reactor Design Single Reaction in Batch, CSTR, PFR 2a. Levenspiel Plots (Reactor Sizing)Document12 pagesCHEE 321: Chemical Reaction Engineering 2. Isothermal Reactor Design Single Reaction in Batch, CSTR, PFR 2a. Levenspiel Plots (Reactor Sizing)Pranav NakhateNo ratings yet
- Sample Exams Problems CHE 402Document3 pagesSample Exams Problems CHE 402Ricardo VelozNo ratings yet
- Conversion and Reactor SizingDocument27 pagesConversion and Reactor SizingJam imtiazNo ratings yet
- Practice Problem Set 2Document1 pagePractice Problem Set 2oprudra2000No ratings yet
- Review Kinetics 1Document70 pagesReview Kinetics 1Ricky JayNo ratings yet
- Cre - 1Document10 pagesCre - 1dhavalNo ratings yet
- Microsoft Word - 6 - Prob RTD-Non Id React 11-12 61-78 - EnglishDocument9 pagesMicrosoft Word - 6 - Prob RTD-Non Id React 11-12 61-78 - EnglishPavithra Sivaraja100% (1)
- CN2116 QZ1Document31 pagesCN2116 QZ1Wang ShenghaoNo ratings yet
- KRD Chapter 2Document39 pagesKRD Chapter 2Reyhan97No ratings yet
- Wollo University Kombolcha Institute of Technology: Chemical Engineering Department Reaction Engineering IDocument41 pagesWollo University Kombolcha Institute of Technology: Chemical Engineering Department Reaction Engineering ITalew TadesseNo ratings yet
- Tubular Flow Reactor Sample UiTM Lab ReportDocument20 pagesTubular Flow Reactor Sample UiTM Lab ReportNur AqilahNo ratings yet
- Advanced Chemical Reaction EngineeringDocument1 pageAdvanced Chemical Reaction EngineeringIbmWasuserNo ratings yet
- Batch CSTR ExperimentDocument5 pagesBatch CSTR ExperimentNaeem YounisNo ratings yet
- BNBBHBHBHJBJHDocument25 pagesBNBBHBHBHJBJHZati TarhiziNo ratings yet
- FTW1Document52 pagesFTW1pubgkrpg09No ratings yet
- Lecture 3 Part 1 PFRDocument14 pagesLecture 3 Part 1 PFRAhmadYossryMuham'madNo ratings yet
- CRE IdocxDocument8 pagesCRE IdocxParth DesaiNo ratings yet
- Mole Balances: Prepared by DR Lee Kiat MoonDocument18 pagesMole Balances: Prepared by DR Lee Kiat MoonfjasfNo ratings yet
- CH142L - Experiment 1Document17 pagesCH142L - Experiment 1Allyssa BadilloNo ratings yet
- CH3010 2010Document1 pageCH3010 2010deepakNo ratings yet
- CHE3044F, 2013: Reactor Design 1: TUTORIAL 2Document2 pagesCHE3044F, 2013: Reactor Design 1: TUTORIAL 2nmhatityeNo ratings yet
- Practice Problem-1Document1 pagePractice Problem-1harshraj.ecellNo ratings yet
- Computational NeuroendocrinologyFrom EverandComputational NeuroendocrinologyDuncan J. MacGregorNo ratings yet
- Simulation of Some Power Electronics Case Studies in Matlab Simpowersystem BlocksetFrom EverandSimulation of Some Power Electronics Case Studies in Matlab Simpowersystem BlocksetRating: 2 out of 5 stars2/5 (1)
- Simulation of Some Power Electronics Case Studies in Matlab Simpowersystem BlocksetFrom EverandSimulation of Some Power Electronics Case Studies in Matlab Simpowersystem BlocksetNo ratings yet