Professional Documents
Culture Documents
Tutorial Chapter 6-7 PDF
Tutorial Chapter 6-7 PDF
Uploaded by
nanaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Tutorial Chapter 6-7 PDF
Tutorial Chapter 6-7 PDF
Uploaded by
nanaCopyright:
Available Formats
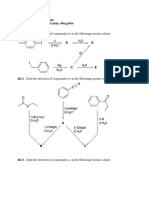
Tutorial Chapter 6 & 7
1. Show the structures of the following amino acids in their zwitterionic forms:
a) Trp
b) Ile
c) Cys
d) His
2. Proline has pKa1 = 1.99 and pKa2 = 10.60. Use the Henderson–Hasselbalch equation
to calculate the ratio of protonated and neutral forms at pH 2.50. Calculate the ratio of
neutral and deprotonated forms at pH 9.70.
3. Using both three- and one-letter codes for amino acids, write the structures of all
possible peptides containing the following amino acids:
a) Val, Ser, Leu
b) Ser, Leu2, Pro
4. Write full structures for the following peptides:
a) C-H-E-M
b) P-E-P-T-I-D-E
5. Identify the monomer units from which each of the following polymers is made, and tell
whether each is a chain-growth or a step-growth polymer.
6. Draw the structure of Kodel, a polyester prepared by heating dimethyl 1,4-
benzenedicarboxylate with 1,4-bis(hydroxymethyl)cyclohexane.
You might also like
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- 4.4 Exercise 2 - IsomerismDocument1 page4.4 Exercise 2 - IsomerismMai Chi0% (1)
- A1 Amino AcidsDocument6 pagesA1 Amino AcidsGabby Tanaka0% (2)
- Chemistry Unit 4 Goodie BagDocument29 pagesChemistry Unit 4 Goodie BagJacob Salkin100% (2)
- CHM 102Document4 pagesCHM 102Fumzy AdelakunNo ratings yet
- OC Part B QuestionsDocument10 pagesOC Part B QuestionsSunanda 2004No ratings yet
- Tutorial 3 NSC 2410 Unit 2 Biomolecules Proteins JULY 2021Document3 pagesTutorial 3 NSC 2410 Unit 2 Biomolecules Proteins JULY 2021Boyd benson kayomboNo ratings yet
- Subjective Test IsomerismDocument2 pagesSubjective Test IsomerismSanjay Mani TripathiNo ratings yet
- PROBLEM SET 1 - Amino Acids and PeptidesDocument3 pagesPROBLEM SET 1 - Amino Acids and PeptidesAnnabella Quevedo CampomanesNo ratings yet
- Organic Chemistry Exam 1 (Practice) Chem 237Document3 pagesOrganic Chemistry Exam 1 (Practice) Chem 237Ngoc Minh NgoNo ratings yet
- Ass.2 MayDocument4 pagesAss.2 Mays9n9No ratings yet
- Q2 2022 OrgchemDocument2 pagesQ2 2022 OrgchemHazeljoyce AlcantaraNo ratings yet
- Problem Set 5 PDFDocument2 pagesProblem Set 5 PDFLouisNo ratings yet
- CHM133 PRELIM EXAM Part2Document7 pagesCHM133 PRELIM EXAM Part2Rohaisa FaisalNo ratings yet
- Chemistry 101 Practice EXAM ANSWERSDocument25 pagesChemistry 101 Practice EXAM ANSWERSSakinah AzmiNo ratings yet
- Practice - Organic Nomenclature: Directions: Write Your Answers To The Following Questions in The Space Provided. UseDocument8 pagesPractice - Organic Nomenclature: Directions: Write Your Answers To The Following Questions in The Space Provided. UseKeshav GuptaNo ratings yet
- TD Bio 211 2023-2024Document9 pagesTD Bio 211 2023-2024Remadji vieriNo ratings yet
- Attachment 1 - 2024-02-21T191802.458Document15 pagesAttachment 1 - 2024-02-21T191802.458copadmmmmNo ratings yet
- Chapter 5 - Biomolecules: Amino Acids, Peptides, and ProteinsDocument3 pagesChapter 5 - Biomolecules: Amino Acids, Peptides, and ProteinsAmrun RusrlNo ratings yet
- 11 GocDocument2 pages11 GocHarsh SinghNo ratings yet
- Tutorial Chapter 3Document1 pageTutorial Chapter 3ShahrizatSmailKassimNo ratings yet
- ChemX6X MT 1Document8 pagesChemX6X MT 1Phương Nail TócNo ratings yet
- Problem Set 5 Solution PDFDocument3 pagesProblem Set 5 Solution PDFLouisNo ratings yet
- CH105 - (O) - Tutorial3 (Q) PDFDocument2 pagesCH105 - (O) - Tutorial3 (Q) PDFShivansh SinghNo ratings yet
- Chapter 4 QuestionsDocument2 pagesChapter 4 Questionsdaniday1977100% (1)
- MCQ On Molecular BiologyDocument12 pagesMCQ On Molecular Biologyronojoysengupta0% (1)
- Chem 112A Homework 1Document4 pagesChem 112A Homework 1Shyam BhaktaNo ratings yet
- SCH 2202 Organic Chemistry IiDocument3 pagesSCH 2202 Organic Chemistry Iimichael100% (1)
- Alkanes - WorksheetDocument2 pagesAlkanes - WorksheetDoug GilmourNo ratings yet
- OrganicDocument6 pagesOrganicTrent SwordsNo ratings yet
- Taller de ESTEREOQUIMICADocument6 pagesTaller de ESTEREOQUIMICAFrancisco Alejandro Rosero SeguraNo ratings yet
- Organic Chem ExDocument15 pagesOrganic Chem Exwan zhouNo ratings yet
- Final Exam Review Fall 2009 AnswersDocument14 pagesFinal Exam Review Fall 2009 AnswersCharisma SubaNo ratings yet
- 3.1.1 Naming Practice QuestionsDocument33 pages3.1.1 Naming Practice QuestionsAyaan RaufNo ratings yet
- Chap 15-24Document49 pagesChap 15-24Linh NguyễnNo ratings yet
- Test 4Document24 pagesTest 4TomNo ratings yet
- Vidyasagar University: B.Sc. Honours Examination 2021Document5 pagesVidyasagar University: B.Sc. Honours Examination 2021Bhawana SharmaNo ratings yet
- Tutorial 2 @conformation PDFDocument3 pagesTutorial 2 @conformation PDFMoulindu KunduNo ratings yet
- CH1O3 Questions PDFDocument52 pagesCH1O3 Questions PDFPrince T MashandaNo ratings yet
- Chem 0000Document5 pagesChem 0000chikondikosamu24No ratings yet
- Biomacromolecules QpsDocument4 pagesBiomacromolecules QpsAshutosh MallickNo ratings yet
- Chemistry QP - FYDocument2 pagesChemistry QP - FYmuneerkkmullaNo ratings yet
- B, Best Depicts Pyridine N-Oxide? Why?Document2 pagesB, Best Depicts Pyridine N-Oxide? Why?pNo ratings yet
- Chemistry (S) Paper VISDocument2 pagesChemistry (S) Paper VISJashanjib MukhopadhyayNo ratings yet
- Biochemistry 244-Midterm 1 2014Document12 pagesBiochemistry 244-Midterm 1 2014DanielNo ratings yet
- 2423 Exam1Document9 pages2423 Exam1Ricardo SierraNo ratings yet
- Chem52 Su13 PracticeExam1ADocument11 pagesChem52 Su13 PracticeExam1Aamarka01No ratings yet
- TS% Acide Alpha Amine%Document3 pagesTS% Acide Alpha Amine%Ibrahima DiopNo ratings yet
- 3.091 Introduction To Solid-State Chemistry - Fall 2012 Problem Set 12 Problem Set Quiz To Be Held December 6Document3 pages3.091 Introduction To Solid-State Chemistry - Fall 2012 Problem Set 12 Problem Set Quiz To Be Held December 6Truong CaiNo ratings yet
- 1021 Workshop W3Document7 pages1021 Workshop W3Gavin DingNo ratings yet
- Chem Exam 4Document30 pagesChem Exam 4TomNo ratings yet
- 13 DPP 25l Goc Excel Mixed (Acid+Base+Dipole - 4-Sub)Document4 pages13 DPP 25l Goc Excel Mixed (Acid+Base+Dipole - 4-Sub)Technology MediaNo ratings yet
- Alkanes Cycloalkanes and AlkenesDocument3 pagesAlkanes Cycloalkanes and AlkenesDorota ZębikNo ratings yet
- CHEM 120N: Organic Chemistry Exam (Exam 2) 45 Multiple ChoiceDocument4 pagesCHEM 120N: Organic Chemistry Exam (Exam 2) 45 Multiple Choicehumba33No ratings yet
- 2013 Homework KimorDocument23 pages2013 Homework KimorDiamondNadinaNo ratings yet
- CHE 232 Test 1Document11 pagesCHE 232 Test 1moatlhodiNo ratings yet
- CHEM1280 2011 Midterm Exam PDFDocument3 pagesCHEM1280 2011 Midterm Exam PDFLouisNo ratings yet
- Chem PPRDocument4 pagesChem PPRJitendra KaushikNo ratings yet
- The Peptides: Volume II Synthesis, Occurrence, and Action of Biologically Active PolypeptidesFrom EverandThe Peptides: Volume II Synthesis, Occurrence, and Action of Biologically Active PolypeptidesNo ratings yet