Professional Documents
Culture Documents
Hidrolisis
Uploaded by
N0 ratings0% found this document useful (0 votes)
31 views1 pagereaciones
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentreaciones
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
31 views1 pageHidrolisis
Uploaded by
Nreaciones
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
Write the hydrolysis reaction(s) for each of the following substances.
(I didn’t ask you to predict whether the resulting solution was acidic or basic, but am
providing that answer anyway.)
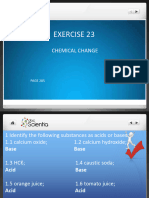
1. Ba(NO3)2 → Ba2+ + 2 NO3-
No further reactions, resulting solution is neutral.
2. Li3PO4 → 3 Li+ + PO43-
PO4-3 + H2O <=> HPO42- + OH-
Resulting solution is weakly basic.
3. HClO4 + H2O → H3O+ + ClO4- OR (Not both!) HClO4 → H+ + ClO4-
Resulting solution is strongly acidic.
4. NaCH3CO2 → Na+ + CH3CO2-
CH3CO2- + H2O <=> CH3COOH + OH-
Resulting solution is weakly basic.
5. C5H5N + H2O <=> C5H5NH+ + OH-
Resulting solution is weakly basic.
6. C5H5NHClO4 → C5H5NH+ + ClO4-
C5H5NH+ <=> H+ + C5H5N OR C5H5NH+ + H2O <=> H3O+ + C5H5N
Resulting solution is weakly acidic.
7. KCN→ K+ + CN-
CN- + H2O <=> HCN + OH-
Resulting solution is weakly basic.
8. NaNO2 → Na+ + NO2-
NO2- + H2O <=> HNO2 + OH-
Resulting solution is weakly basic.
9. NH4Cl → NH4+ + Cl-
NH4+ <=> H+ + NH3 OR NH4+ + H2O <=> H3O+ + NH3
Resulting solution is weakly acidic.
10. CaF2 → Ca2+ + 2 F-
F- + H2O <=> HF + OH-
Resulting solution is weakly basic.
Note: Stoichiometric coefficients only balance the equation they are in. They do not carry
through to subsequent reactions!
You might also like
- List of Irreversible ReactionsDocument12 pagesList of Irreversible Reactionscecil3414No ratings yet
- Asam Basa - 2 PDFDocument6 pagesAsam Basa - 2 PDFRendy Dwi JuliasmanNo ratings yet
- Reactions in Aqueous SolutionDocument64 pagesReactions in Aqueous SolutionSoul Relaxation LabNo ratings yet
- Ionic EquilibriaDocument10 pagesIonic EquilibriaShantanu KadamNo ratings yet
- Chapter 5 Acids Base EquilibriaDocument105 pagesChapter 5 Acids Base Equilibriantranh58No ratings yet
- Salt HydrolysisDocument16 pagesSalt HydrolysisJoseph Marlon ClaroNo ratings yet
- CH186 Acid-Base Exam Questions From Spring 2001 SemesterDocument6 pagesCH186 Acid-Base Exam Questions From Spring 2001 SemesterArda RahmainiNo ratings yet
- UP CHEM 31 Alcohols, Ethers, and PhenolsDocument2 pagesUP CHEM 31 Alcohols, Ethers, and PhenolsZsara CampanoNo ratings yet
- Acid Base Equilibria and Buffer SolutionsDocument27 pagesAcid Base Equilibria and Buffer SolutionsDavidson ChanNo ratings yet
- 09Document12 pages09ZenPhiNo ratings yet
- Organic Functional Group Tests - Practicals Chemistry Class 12Document4 pagesOrganic Functional Group Tests - Practicals Chemistry Class 12Rudraksh mittalNo ratings yet
- Chap 5 Part 4 AcidsBases - PH Salts (46-59)Document14 pagesChap 5 Part 4 AcidsBases - PH Salts (46-59)gbygbybNo ratings yet
- Complex Acid-Base Systems PDFDocument35 pagesComplex Acid-Base Systems PDFHannah CenaNo ratings yet
- Aqueous Solution ChemistryDocument9 pagesAqueous Solution ChemistryDilekNo ratings yet
- National Academy For Learning Bengaluru 2021-2022 Grade 10 ICSE Chemistry Practical HandoutDocument18 pagesNational Academy For Learning Bengaluru 2021-2022 Grade 10 ICSE Chemistry Practical HandoutpranavNo ratings yet
- Report - Group 5Document27 pagesReport - Group 5Quỳnh Võ Nguyễn NhậtNo ratings yet
- Sec 4.13 - Hydrolysis (Notes) : Group 1 (Alkali Metal Ions) Eg. Li Group 2 (Alkaline Earth Ions) Eg. BeDocument15 pagesSec 4.13 - Hydrolysis (Notes) : Group 1 (Alkali Metal Ions) Eg. Li Group 2 (Alkaline Earth Ions) Eg. BeDavid SobralNo ratings yet
- Reactions in Aqueous SolutionsDocument83 pagesReactions in Aqueous Solutions張婷昀No ratings yet
- Chemistry 1A Fall 2006 Exam 2 Key Chapters 4, 5, 6, and 7 (Part)Document6 pagesChemistry 1A Fall 2006 Exam 2 Key Chapters 4, 5, 6, and 7 (Part)Anonymous oTrMzaNo ratings yet
- Van't Hoff FactorDocument17 pagesVan't Hoff FactorRaymond Godfrey Dagwasi100% (1)
- Acid Bases and Salts MCQ QuestionsDocument10 pagesAcid Bases and Salts MCQ Questions09whitedevil90No ratings yet
- Acid Base 2006Document24 pagesAcid Base 2006Vina SoumokilNo ratings yet
- Chapter 9Document33 pagesChapter 9helloblarg100% (4)
- Chemistry Chapter 2Document160 pagesChemistry Chapter 2Deepak vishalNo ratings yet
- Practical Organic ChemistryDocument10 pagesPractical Organic ChemistryMukundNo ratings yet
- Acid Base CH 16 ComprehensiveDocument4 pagesAcid Base CH 16 ComprehensiveAidah AmirNo ratings yet
- Acid Base Equilibrium Practice TestDocument3 pagesAcid Base Equilibrium Practice Testapuszis100% (2)
- Chapter4-Kesetimbangan Asam BasaDocument115 pagesChapter4-Kesetimbangan Asam BasatitoNo ratings yet
- Acid BaseDocument63 pagesAcid BaseFrian LiaNo ratings yet
- Waste ProcessDocument86 pagesWaste ProcessacidoanimalNo ratings yet
- AP Chem Unit 8Document38 pagesAP Chem Unit 8asudeeeNo ratings yet
- 13-Acids and BasesDocument44 pages13-Acids and BasesShamier Khent SamsonNo ratings yet
- Acid Base Theory AnswersDocument4 pagesAcid Base Theory AnswersPasha TanNo ratings yet
- Namma Kalvi 12th Chemistry 2 Mark and 3 Mark Study Material em 217115Document20 pagesNamma Kalvi 12th Chemistry 2 Mark and 3 Mark Study Material em 217115Loyal FriniteNo ratings yet
- Worksheet Chapter 4.I ANSWERSDocument2 pagesWorksheet Chapter 4.I ANSWERSkhalid badranNo ratings yet
- Chemistry 2 Physical Chemistry Lecture NotesDocument48 pagesChemistry 2 Physical Chemistry Lecture Noteskittycat1chauNo ratings yet
- Chapter5-Kesetimbangan Asam BasaDocument115 pagesChapter5-Kesetimbangan Asam BasaAnnisah MardiyyahNo ratings yet
- Saint Mary's University: Quiz On Balancing of Chemical EquationsDocument2 pagesSaint Mary's University: Quiz On Balancing of Chemical EquationsJuliannaViktoriiaNo ratings yet
- NS1Lec - Module 5 - NacionalesDocument5 pagesNS1Lec - Module 5 - NacionalesWindere Marie NacionalesNo ratings yet
- Chemistry Form 6 Sem 3 10Document29 pagesChemistry Form 6 Sem 3 10Anonymous WAnr0jvNo ratings yet
- Aqueous Solution and Chemical EquilibriaDocument27 pagesAqueous Solution and Chemical EquilibriaS. MartinezNo ratings yet
- Redox Tirtations Word.Document21 pagesRedox Tirtations Word.tabbykaranja080No ratings yet
- Chem 31.1 Expt 1. SolubilityDocument3 pagesChem 31.1 Expt 1. SolubilityBuiHope100% (2)
- Botp - Basic ChemistryDocument23 pagesBotp - Basic ChemistryjtichemicalNo ratings yet
- Preliminary Instructions: Cu Ni CR Fe FeDocument4 pagesPreliminary Instructions: Cu Ni CR Fe FeEmmanuel Ryan100% (1)
- Ade 11 Chemistry Exercise 23 Page 285Document9 pagesAde 11 Chemistry Exercise 23 Page 285khanyisan60No ratings yet
- Oganic Cem PDFDocument2 pagesOganic Cem PDFaulaNo ratings yet
- H2 Revision Notes For Promo 2022 (Lecture Notes Answers)Document28 pagesH2 Revision Notes For Promo 2022 (Lecture Notes Answers)22S35 TIOH JING KAINo ratings yet
- Balancing Chemical EquationsDocument19 pagesBalancing Chemical EquationsTrapsetNo ratings yet
- Ionic Equilibrium Lecture-13 Notes PDFDocument10 pagesIonic Equilibrium Lecture-13 Notes PDFnimit singhNo ratings yet
- EMAN YASSIN - Chemistry 12 Unit 4 PacketDocument11 pagesEMAN YASSIN - Chemistry 12 Unit 4 PacketSherine GamalNo ratings yet
- Balancing EquationsDocument81 pagesBalancing Equationsbrian.kuzakNo ratings yet
- Chapter 4 Reactions in Aqueous SolutionDocument90 pagesChapter 4 Reactions in Aqueous SolutionFABIO DE LIMANo ratings yet
- 18 silberberg8eISMChapter18 9eDocument68 pages18 silberberg8eISMChapter18 9efgb9qfb7x6No ratings yet
- Inorganic Chemistry Acids & Bases: Pauling'sDocument6 pagesInorganic Chemistry Acids & Bases: Pauling'sAlmasriJosephNo ratings yet
- KBR NH No Alcl Na Hpo: Chem 116 Fall 2006 Prof. SevianDocument2 pagesKBR NH No Alcl Na Hpo: Chem 116 Fall 2006 Prof. SevianLucasLeãoNascimentoNo ratings yet
- Balancing Chemical EquationsDocument2 pagesBalancing Chemical EquationsBRADEN LEWISNo ratings yet
- Note - Acid and BaseDocument3 pagesNote - Acid and BaseAnwar FadilNo ratings yet