Professional Documents
Culture Documents
Improvements in The Manufacture and Production of Metals From Metal Halides
Uploaded by
KurtWatley0 ratings0% found this document useful (0 votes)
8 views4 pagesImprovements in the Manufacture and Production of Metals From Metal Halides
Original Title
Improvements in the Manufacture and Production of Metals From Metal Halides
Copyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentImprovements in the Manufacture and Production of Metals From Metal Halides
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
8 views4 pagesImprovements in The Manufacture and Production of Metals From Metal Halides
Uploaded by
KurtWatleyImprovements in the Manufacture and Production of Metals From Metal Halides
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You are on page 1of 4
PATENT SPECIFICATION
Application Date: Feb, 27, 1930, Ne, 6631 |: 30.
Complete Left : Deo, 26, 1980.
Complete Accepted : April 23, 1981.
PROVISIONAL SPECIFICATION,
Improvements in the Manufacture and Production of Metals
from Metal Halides.
1, Janes Yar Joussox, a British Sub-
ject, of 47, Tancoln's Lan Wilds, in tho
County of “London, Gentleman, do
hereby declare the nature of this faven-
5 tion (whieh lag been communicated to mo
from ‘abroad. by. T. G._ Farbenindastrie
ltenzeatha, of Fraaknrtonifain,
Germany. a Joint ‘Stock Company organ
ined under the Lins of Germany) ‘0 be
10 as follows Y
faving regard to the hydrogen, halide
axising in the reduction of metal halides
with hydrogen it has" been customary
Hitherto to constructor coat the apparatus
45 for the said reduction of or with ceramic
anaterial
My foreign correspondents have now
fousd that the reduction aay he carried
ou in a much more convenient manner
ju a metalic, Zor example in an ordinary
iron ‘or stool apparatus, provided that
Ghose parts of the apparatie which come
into contact with the. hydrogen. halide
formed are kapt hot, ‘The employment of
metalic apparatus he great arate
age that the heat necessiry for carry
ot the said reduction can bo directly
supplied through the walls to the reagents
ina convenient manner. ‘The tempera
‘80 tures at which the metallic parts of the
apparatus are to be kept depend on the
Specific metal employed and_the concen-
{ration of the hydrogen hatide formed in
the gus mixture, which may be higher the
Ligier the temperature of the metallic
parts,” has, for example in the case of
fron the hydrogen chloride concentration
sabe ab ih 260, per vent of the
hvdrogen present when the iron parts are
Kept at 700° Centigrade, srhereas the con-
centration of hydrogen chloride may rise
to about B0 per cent. at 000° Centigrade.
Tron parts eoming into. contact with
hydrogen halide should, however, never
bp cooler than about 400° "Centigrade.
Generally speaking, it ip advisable fo'uso
for the construction of the apparatus the
same metal as is to be produced therein,
When employing a nobler metal than that
to be proluceds for example copper for
Apparatus in which fron is. fo be: pro.
diveed, these “metallic parts. through
‘which’ the “heat “fs_not "supplied may
i
45
50
he kept at lower temperatures than
would be neceseary in the case of loss
joble metal theconomtraton, of
iydrogen halide may be higher, ‘The
tiuploymont of melal for the construction
of tie heat conveying parts is of particu.
Jer advantage, sinco the production of
retals from tho corresponding halides, for
Sample the production of on fom iron
fords by means of hydrogen, is in most
tases strongly endothermic and therefore
diffeatties atise when employing ceramic
taterial for the construction of the appa-
Totus a5 was usual hitherto, These dif
callie are obviated. according’ to the
Present invention by heating the reaction
Chamber hy means of heating devices con-
structed of metal. Any ris of the metal
being corroded by an attack of hydrogen
is much lessened by employing metals as
Dare as possible, as for example iron free
from caivon. ‘ihe following metals may,
for example, be employed for the con:
struction of the apparatus, copper, in,
nickel, cobalt, “ehromium, end’ their
halides may be reduced.” As. further
Iietal halides which may be reduced by
means of hydrogen. in apparatus con
structed in accordance. with the present
Enventjon inay be mentioned zine clorde,
molybdenum’ chloride and tungsten
chloride,
The following Examples will further
‘Mluatrate the nature of this invention, but
the invention is not resrcted to. these
Examples.
Exporter 1
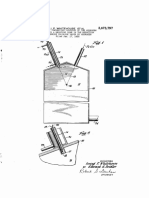
6 kilograms of pure “sublimed iron
chloride are charged into a wrought. iron
tube, 1600 millimetres im length and 150
millimetres in internal diameter. In the
tubo, which may be heated externally by
meats of electrical resistance heating, js
arranged a second iron tube 70. milli-
sotres in internal diameter in which like-
‘wise an electrical resistance heating ele-
ment is embedded. By leading for about
20 hours a current of 200 litres of hydro-
gen per hour through the outer tube and
Simultaneously heating the outer take to
fan internal wall temperature of 600°
Centigrade and the inner tube to an
external wall temperature of 700° Centi-
346,921
90
105
346,921
‘grade, the outer tube becomes coated with
8 homogeneous, finely-orystalline layer of
pure iron, while the greater part of the
Hon sopaates in 9 more coherent form,
5 parily in exystals soveral, contimotres in
See, on the inner heating tube, The thin
coating layer on the outer tube isallowed to
remain, the inner tube is withdrawn, the
greater part of thé iron (about 2 kilo-
grams) is removed therefrom, the appa-
Fatus rouegumbld and fled with 9 fech
charge. By working in this manner no
comrosion whatever of the wrought iron
paris of the apparatus takes place. ‘The
45 excess of hydrogen employed during the
yeduction and which escapes with the
hydrochloric acid formed is freed from the
Iatier in the manner alveady known by
washing with water, dried and retuned
fo the process,
Exar 2
10 kilograms of chromous chloride are
heated at 950° Centigrade in a revolving
ron, drum heated externally by means of
heating gases while passing” hydro
through the said drum at a rate of 2¢
40
ubio metres per hour. 4050. grams of
chromium in the form of fine needles are
sbiaiued in 800 hours. ‘The chromium
obiained is of great purity, in particular
1 fee fron cabo and therefore itis
especially suitable for the production o
chromium alloys, for example acid-proot
steels and the Vike
Exam: 3
Cuprous chloride is continuously passed
at a rate of 10 kilograms per 2 hours
throagh a revolving iron furnace heated
externally by electric means, and heated
at 450° Centigrade im a current of hydro-
gen passed through the said furnace in
the opposite direction ata rate of 5.5,
cubic metres per hour. ‘The issuing gases
are led away by means of tuhes of ceramic
or similar acid-proot material. The
copper is obtained am pulverulent amor-
‘phous form and in a state of great purity.
Dated this 27th day of Bubruary, 1930,
J. ¥, & GW. JOHNSON,
47, Lincoln's Tan Fils, London, W.C. 2,
COMPLETE SPECIFICATION.
Improvements in the Manufacture and Production of Metals
from Metal Halides.
I, Jaxms Yars Jorrson, a British Sub-
ject, of 47, Tincoln’s Inn Fields, in the
jounty of London, Gentleman, do
hhereby declare the nature of this iaven-
tion (Which has been communicated to me
from abroad. by I. G, Farbenindustrie
Alctiengesellechaft, of Frankfort-on-Main,
Germany, a Joint’ Stock Company organ:
ized under the Laws of Germany) and in
what manner the same is to be performed,
to he particularly described and ascer
tained’ in and by the following state-
‘Having regard to the hydrogen halide
arising in the reduction of metal bolides
with hydrogen it has been customary
hitherto to construct or coat the apparatus
for the said reduction of or with ceramic
matarial,
My foreign correspondents have now
found that the reduction may be carried
out in a much more convenient manner
in an apparatus constructed of ordinary
iron suck as, for example steel, east iron
or wrought iron, or iron alloys ‘consisting
mainly of iron such as for example nickel
or chrome steels, provided that those parts
"75 of the apparnius whieh come into contact
with the hydroren halide formed are kept
hot. ‘The employment of iron apparatus
‘has the great advantage that the heat
50
5
60
0
necessary for carrying out the said redue~
Bee aa geared veg
wally to tho reagents ina convenient
Insnner, ‘The temperatures at which the
iron parts of the apparatus are to be kept
depend on the concentration of the hydro-
fen halide formed. inthe. gas misture,
‘which may be higher, the higher the tem
Deraiure of the iron parts, ‘Thus, for
Gxamaplo the hytrogen chloride eoneentrac
tion may be as hugh as 20 per cent. of
the hydrogen present when te iron parts
are kept at 100° Centigrade, ‘whereas the
Concentration of hydrogen chloride ma
Sino sbgut 6 pe cent a. 900 Cont
je, Tron pars coming into contact
Fith hydrogen halide. should, however,
nares Cree an eeet Fens
grade. The employment of iron for the
onstruction of the heat, conveying” parts
{S of particular advantage, since the pro-
duction of metals from the cotresponding
huaides, “for example, ‘the production
of iton from iron eiloride ‘by means
of hydrogen, isin most cases stronaly
endothermic “and. therfore difleulies
frit. when employing. eramie material
for the consiruclion of the appt-
rafus as wae usual hitherto, ‘These dite
bullies aro obviated according to the
present invention by heating the reaction
105
346,921 3
chomber by means of heating devices con-
structed of iron. Any risk of the iron
being corroded by an attack of hydrogen
is much lessened by employing iron oF
5 iron alloys as free as possible from sub-
stituents which are removed by the action
of hydrogen, as for example carbon, sul-
phur and phosphorus. ‘The halides of the
following metals may for example, be
40 reducolin the iron’ apparatus, copper,
fron, nickel, cobalt, “chromium, snc,
molybdenum and tungsten.
‘The following Examples will further
illustrate how the invention is carried out
15 in practice, but the invention is not re-
stricted ta ‘these Examples.
‘Exacres I.
6 kilograms of pure’ ‘sublimed iron
chloride are charged into a wrought iron
tube 1500 millimetres im longth and 150
nillimetres in internal diameter. In the
tube, which may be heated extemally by
meats of electriea) resistance heating, is
arranged a second iron tube 70_milli-
aietres in internal diaineter in which like-
‘wise an eleotrical resistance heating ele-
ment is embedded, By leading for about
20 hours a current of 200 litres of hydro-
zen per hour throngh the outer tube and
30 simultaneously heating the cuter tube to
an internal wall temperature of 600°
Centigrade and the immer tube to an
external wall temperature of 700° Centi-
rade, the outer tube becomes coated with
35 a homogeneous, finely-crystalline layer of
pure iron, while the greater part of the
tron, separates in a more coherent form,
partly in crystals several centimetres in
size, on the inner heating tube. The thin
coating layer on theouter tube isallowed to
remain, the inner tube is withdrawn, the
greater part of the iron, (about. 2 kilo-
rams) is removed therefrom, the appa-
‘plus reeled au llag with froth
charge. By working in this manner no
corrosion whatever of the wrought ixon
parts of the apparatus takes place... ‘The
excess of hydrogen employed during the
reduction and which escapes with the
hydrochloric acid formed is freed from the
latter in the manner already known by
washing with water, dried and retuned
to the process.
‘Bsanene 2
10 kilograms of chromous chloride are
heated at 950° Centigrade in a revolving
iron drum heated externally by means of
Theating gases while passing hydrogen
through the said drum sta vate of 20
60 cubic metres per hour. 4050 grams of
chromium in he form of fine needles are
obtarned in 300 hours. The ehromium
obtained is of great purity. in particular
it is free from carbon, and therefore it is
65 especially suitahie for the production of
50
85
chromium alloys, for example acid-proof
stele onl the tke
Exon 3
Cuprous chloride is continuously passed
at a rate of 10 kilograms. per 2 hours
through a revolving iron furnace heated
externally by electric means, and heated
at 450° Centigrade in a current of hydro-
gen passed through the said furnace in
the opposite direction at a vate of 5.
cubie metres per hour. ‘The issuing gases
are led away by means of tubes of ceramic
or similar’ acid-proof material. The
coprer is obtained in pulverulent_amor-
phous form and an a state of great purity.
7 kilograms of pure ferrous chloride ave
filed into an ison tube of 1500 millimetres
Iength and 150° millimetres internal
diameter, the inner wall of the said tube
being provided with a thin layer of
quarts, In the tube is arranged a second
fron tube of 65. millimetres internal
diameter which is heated at an external
temperature of about 700° Centi-
grade by burning a mixture of carbon
monoside and osygen in the tube, On
passing hydrogen preferably preheated by
means of the issuing hot gases for 24
hours over ihe ferrous chloride at a rate 95
of 200 litres per hour the ferrous chloride
fs reduced and the bulk of the iron
formed, about 3 kilograms, is, deposited
on the inner tube and may be discharged
therefrom after withdrawing the inner
tue.
Hating may also be effected by a flame-
loss surface combustion of heating: gases
in the inner tube.
400
Having now particularly deseribed and
ascertained the nature of my said inven-
tion and in what manner Ue same is
{o Be performed, T declare that what 1
1. Tn the manufacture and production
of metals by reduction of their halides by
means of hydrogen, carrying out the
reduction in au apparaty: of which at
least the heat conveying parts are made of
iron, or iron alloys consisting mainly of
iron kept at a temperatnre of at least 400°
Centigrade,
2, Apparatus for the manufacture and
production of metals by rednetion of their
halides by means of hydrogen of which at 420
Teast the heat conveying parts axe made
of fron, or iron alloys consisting mainly
3, The process for the manufacture and
production of metals substantially as de-
seribed in each of the foregoing
Examples.
‘4. Metals when prepared in accordance
with the preceding claiming clauses,
105
410
115
125
346,921
J, ¥, & GW. JOHNSON,
‘Dated this 28rd day of December, 1930, 47, Lincoln's Inn Fields, London, W.C. 2,
Agents,
Ueedhill: Printed for Hie Majesty's Stationery OlBoe, by Tare & Maleomson, Ltd—1981
You might also like
- Method of Preventing Clogging of The Hydrogen Inlet To A Reducing Zone in The Reduction of Ferrous Chloride Vapor by HydrogenDocument7 pagesMethod of Preventing Clogging of The Hydrogen Inlet To A Reducing Zone in The Reduction of Ferrous Chloride Vapor by HydrogenKurtWatleyNo ratings yet
- Metastable Soot CPLDocument6 pagesMetastable Soot CPLKurtWatleyNo ratings yet
- A Comment Upon The Correlation of Inductively Coupled Plasma Emission Signal Variations With Spray Chamber Pressure VariationsDocument1 pageA Comment Upon The Correlation of Inductively Coupled Plasma Emission Signal Variations With Spray Chamber Pressure VariationsKurtWatleyNo ratings yet
- !spinning of Carbon Nanotube Fibres Using The Floating Catalyst High Temperature Route Purity Issues and The Critical Role of SulphurDocument19 pages!spinning of Carbon Nanotube Fibres Using The Floating Catalyst High Temperature Route Purity Issues and The Critical Role of SulphurKurtWatleyNo ratings yet
- Methods For Synthesis of NanoparticlesDocument20 pagesMethods For Synthesis of NanoparticlesKurtWatleyNo ratings yet
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)