Professional Documents
Culture Documents
NMR of Carbon (AQA A2 Chemistry) PART 2 of 2 TOPICS
NMR of Carbon (AQA A2 Chemistry) PART 2 of 2 TOPICS
Uploaded by
muhammad shehariyarOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
NMR of Carbon (AQA A2 Chemistry) PART 2 of 2 TOPICS
NMR of Carbon (AQA A2 Chemistry) PART 2 of 2 TOPICS
Uploaded by
muhammad shehariyarCopyright:
Available Formats
NMR of carbon (AQA A2 Chemistry) PART 2 of 2 TOPICS
Carbon NMR:
Carbon NMR is the same as hydrogen NMR however you look at the different carbon environments
using carbon-13.
The basics that should be known of both types of NMR is covered in another of my documents called
'NMR of Hydrogen'.
Example:
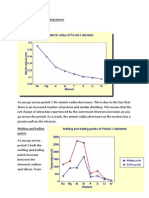
How many different types of peaks would you expect to see using the carbon-13 NMR for this
molecule?
Notice that there are two different carbon environments indicated by the colours
blue and red.
Only two peaks will be appear on the graph where the red peak will be further
away from Si(CH3)4 than the blue peak because the carbon circled in red is closer to
an electronegative element (oxygen).
The peaks will not split like the peaks for the hydrogen NMR because of carbon-13
atoms are so low that it is unlikely that splitting will happen. If it did, it would be too
small to detect.
This is why carbon-13 NMR is much more simpler than hydrogen NMR.
You might also like
- Concerning Amines: Their Properties, Preparation and ReactionsFrom EverandConcerning Amines: Their Properties, Preparation and ReactionsRating: 2.5 out of 5 stars2.5/5 (2)
- Carbon-13 NMR: Mr. Faheem Shaikh. (M.Pharm 1 SEM.)Document18 pagesCarbon-13 NMR: Mr. Faheem Shaikh. (M.Pharm 1 SEM.)Niraj GuptaNo ratings yet
- Descriptive Inorganic Chemistry 6th Edition Rayner Canham Solutions Manual 1Document5 pagesDescriptive Inorganic Chemistry 6th Edition Rayner Canham Solutions Manual 1richard100% (35)
- CarbonDocument1 pageCarbonVeena VipinkumarNo ratings yet
- C 13nmr 200309121906Document21 pagesC 13nmr 200309121906ابراهيم الاسطىNo ratings yet
- Spectroscopy ManualDocument21 pagesSpectroscopy Manualanthor100% (2)
- What Is CDocument8 pagesWhat Is Csteven_agassiNo ratings yet
- C-13 NMR and DEPTDocument41 pagesC-13 NMR and DEPTV G Viju Kumar100% (1)
- Carbon NMRDocument3 pagesCarbon NMRPraveenaNo ratings yet
- 22 and Applications of C NMR: Subject ChemistryDocument13 pages22 and Applications of C NMR: Subject ChemistrySaurav PaulNo ratings yet
- Chap03 CarbonylsDocument10 pagesChap03 CarbonylsAPLCTNNo ratings yet
- Carbonyls PDFDocument10 pagesCarbonyls PDFMaheshNo ratings yet
- UGSemsterSyllabus Chemistry 6Sem614Chemistry English InorganicOrganicPhysicalChemistryDocument168 pagesUGSemsterSyllabus Chemistry 6Sem614Chemistry English InorganicOrganicPhysicalChemistryAnil GugulothNo ratings yet
- Lock Roup: Chapter-1Document42 pagesLock Roup: Chapter-1Binod TharuNo ratings yet
- The P Block ElementsDocument4 pagesThe P Block ElementsAthulRKrishnanNo ratings yet
- Carbon Nuclear Magnetic Resonance (13C-NMR) SpectrosDocument3 pagesCarbon Nuclear Magnetic Resonance (13C-NMR) SpectrosBhushan AmruteNo ratings yet
- C NMR SpectrosDocument17 pagesC NMR SpectrosAhmad AlShahrourNo ratings yet
- SCHB032 NMR Part2redoDocument29 pagesSCHB032 NMR Part2redoemjayNo ratings yet
- Spectroscopy and Chromatography 1 Unit 4 New SpecificationsDocument11 pagesSpectroscopy and Chromatography 1 Unit 4 New SpecificationsLoh Jun XianNo ratings yet
- Atyabrata Endh MSC Part I (2017-18) Department of Chemistry Berhampur University, Odisha, IndiaDocument17 pagesAtyabrata Endh MSC Part I (2017-18) Department of Chemistry Berhampur University, Odisha, IndiaDr. Md. Ehtesham Ul HoqueNo ratings yet
- Metalcarbonyls 180405141857Document17 pagesMetalcarbonyls 180405141857Biswajit RoutNo ratings yet
- P BlockDocument4 pagesP BlockAmithrajith P ANo ratings yet
- ATOICV1 11 2 Vibrational Spectra of Metal Carbonyls For Bonding and Structure ElucidationDocument16 pagesATOICV1 11 2 Vibrational Spectra of Metal Carbonyls For Bonding and Structure ElucidationAdnan BukhariNo ratings yet
- Solution - Assignment P BLOCK ELEMENTSDocument4 pagesSolution - Assignment P BLOCK ELEMENTSYash KumarNo ratings yet
- C - NMR: Cracking Those Carbons: Parallel, and Those With Axes Pointing in Opposite Directions Are Called AntiparallelDocument6 pagesC - NMR: Cracking Those Carbons: Parallel, and Those With Axes Pointing in Opposite Directions Are Called AntiparallelMusdalifahNo ratings yet
- 13-C NMR-09Document27 pages13-C NMR-09M Nur M. MahmudNo ratings yet
- 13c NMR SpectrosDocument29 pages13c NMR SpectrosKudzai ChidzodzoNo ratings yet
- C-13 NMRDocument29 pagesC-13 NMREnesEmreTaşNo ratings yet
- Periodicity (ANNEX) - CN - STDT1BDocument1 pagePeriodicity (ANNEX) - CN - STDT1BNkemzi Elias NzetengenleNo ratings yet
- 15TH Group ElementsDocument8 pages15TH Group ElementsredoxreactionsNo ratings yet
- Radiocarbon Dating (Carbon 14 Dating)Document9 pagesRadiocarbon Dating (Carbon 14 Dating)joshua josolNo ratings yet
- c13ppt 150515121301 Lva1 App6892Document16 pagesc13ppt 150515121301 Lva1 App6892kiran yaseenNo ratings yet
- 13,14 Group TheoryDocument29 pages13,14 Group TheoryAlkaChoudharyNo ratings yet
- The Periodic Table - Chemical PeriodicityDocument8 pagesThe Periodic Table - Chemical PeriodicityahumanbeinginearthNo ratings yet
- CarbonDocument28 pagesCarbonjosevitorromualdoNo ratings yet
- Syllabus 3006312820200416101137Document15 pagesSyllabus 3006312820200416101137Kamesh dewanganNo ratings yet
- Teori Meda KristalDocument41 pagesTeori Meda KristalGilang Maulana PutraNo ratings yet
- Chem XIIDocument20 pagesChem XIIalchemy15No ratings yet
- Class 12 P - Block ElementsDocument33 pagesClass 12 P - Block ElementsIpsita SethiNo ratings yet
- Carbon FamilyDocument33 pagesCarbon Familyk narayanaraoNo ratings yet
- Element Symbol Property: (He) 2s 2p (Ne) 3s 3p (Ar) 4s 4p (KR) 5s 5p (Xe) 6s 6pDocument22 pagesElement Symbol Property: (He) 2s 2p (Ne) 3s 3p (Ar) 4s 4p (KR) 5s 5p (Xe) 6s 6pMuskan VashistNo ratings yet
- Element Symbol Property: (He) 2s 2p (Ne) 3s 3p (Ar) 4s 4p (KR) 5s 5p (Xe) 6s 6pDocument10 pagesElement Symbol Property: (He) 2s 2p (Ne) 3s 3p (Ar) 4s 4p (KR) 5s 5p (Xe) 6s 6pVarun BansalNo ratings yet
- Metal Carbonyls: Presented by Ondrila Deb MSC (Applied Chemistry) A4450918040Document15 pagesMetal Carbonyls: Presented by Ondrila Deb MSC (Applied Chemistry) A4450918040Ondrila DebNo ratings yet
- NMR Lec 5Document18 pagesNMR Lec 5Fatima AhmedNo ratings yet
- Inorganic Chemistry 2nd Edition (Housecroft) - Copy - 1Document169 pagesInorganic Chemistry 2nd Edition (Housecroft) - Copy - 1firda noer ainiNo ratings yet
- Chapter - 11 The P-Block ElementsDocument10 pagesChapter - 11 The P-Block ElementsManan TyagiNo ratings yet
- UPDATED PBlock ElementsDocument100 pagesUPDATED PBlock ElementsAarohi SharmaNo ratings yet
- The P Block ElementDocument11 pagesThe P Block Elementusingforadditionalpurpose100No ratings yet
- C NMR: SP Carbon Attached Electronegative Atom OCH OCHDocument7 pagesC NMR: SP Carbon Attached Electronegative Atom OCH OCHChaithraMalluNo ratings yet
- Exception: Atomic Radius of Ga Is Less Than That of Al Due To The Presence of Poor Shedding 10d-Electrons in GalliumDocument2 pagesException: Atomic Radius of Ga Is Less Than That of Al Due To The Presence of Poor Shedding 10d-Electrons in GalliumKisha KhuranaNo ratings yet
- Chemistry Winter HomeworkDocument2 pagesChemistry Winter HomeworkryeNo ratings yet
- AminesDocument24 pagesAminesayesha sheikhNo ratings yet
- Notes For Period 3Document4 pagesNotes For Period 3Ben ToNo ratings yet
- Carbon - WikipediaDocument30 pagesCarbon - WikipediaRickresh MNo ratings yet
- P Block Elements, Class 11Document13 pagesP Block Elements, Class 11Ashish kumarNo ratings yet
- 9.0 PeriodicityDocument22 pages9.0 PeriodicitygoverotaropafadzwaNo ratings yet
- Period 3-Sodium To ArgonDocument56 pagesPeriod 3-Sodium To ArgonKumar FongNo ratings yet
- Lecture 11-Gr 14Document19 pagesLecture 11-Gr 14averagestudent838No ratings yet
- Unit - IiiDocument16 pagesUnit - IiiKartik TripurariNo ratings yet