Professional Documents
Culture Documents
Light Scattering
Uploaded by
Angel Quijano ArmengolCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Light Scattering
Uploaded by
Angel Quijano ArmengolCopyright:
Available Formats
Light scattering
and nanoparticles
Metal oxide nanoparticles are finding increasing application in the
preparation of new nanocrystalline materials, with metal oxide
composites being used to confer new electronic, magnetic and
optical properties into material structures. Often these materials are
formulated and processed as slurries or aqueous suspensions. One key
parameter in controlling the properties of such colloidal nanoparticle
systems is their particle size. Light scattering techniques are widely
used for its determination.
Ulf Nobbmann and Ana Morfesis
Malvern Instruments Ltd, Enigma Business Park, Grovewood Road, Malvern, Worcestershire, WR14 1XZ, UK
www.malvern.com
Comparing results from different methods must be approached size distribution on the application in question. When selecting a size
with caution: particle size distributions reported in terms of measurement technique, it is important to appreciate that for non-
volume, number or scattering intensity usually produce vastly spherical materials containing a range of particle sizes, the likelihood
differing results, despite the data having been derived from exactly is that each will produce different results. We seek to explain why
the same physical material. Alongside particle size, another this is the case using the following example of a TiO2 dispersion.
fundamental colloidal characteristic is zeta potential, which can
also be measured rapidly using light scattering techniques. This Experiment
effectively quantifies the parameter controlling electrostatic TiO2 was obtained from Nanophase Technology Corporation, prepared
stabilization, and can be used in formulation development to avoid in Di-H2O adjusted to pH 3.1 at a concentration of 0.01 wt% and
the instability that can result from particle-particle attraction. sonicated in a bath for 30 sec. The sample serves as a model system
for metal oxides and was chosen here because of its availability and
The potential uses for metal oxide nanoparticles are often size ease of use. The sample labelled “Nanotek” was filtered through a
dependent. For example, the UV absorption power of TiO2 particles1 Whatman Anodisk filter (Whatman plc, Maidstone, UK) with pore size
or the use of iron oxide as contrast agents for magnetic resonance of 0.2 μm to remove undispersed aggregates.
imaging (MRI) scans2 are a function of their particle size. Rather than Size measurements were performed using dynamic light scattering
a single size, most ‘real world’ samples contain a range of sizes, and (DLS) on a Malvern Zetasizer Nano-ZS (Malvern Instruments,
the challenge is to characterize and understand the effects of this Malvern, UK). Samples were irradiated with red light (HeNe laser,
52 MAY 2009 | VOLUME 12 | NUMBER 5 ISSN:1369 7021 © Elsevier Ltd 2009
Light scattering and nanoparticles APPLICATIONS
Size Distribution by Intensity
20
15
Intensity (%)
10
0
0.1 1 10 100 1000 10000
Size (nm)
Record 47: TiO2 Nanotek, pH3, 1 Record 48: TiO2 Nanotek, pH3, 2
Record 49: TiO2 Nanotek, pH3, 3
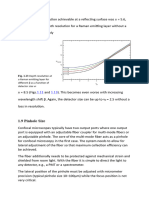
Fig. 1 The intensity size distribution of Nanotek TiO2 versus particle diameter in nanometers is characterized by a peak mean of 85.6 nm. The different colors represent
repeat measurements of the same sample.
wavelength λ = 632.8 nm) and the intensity fluctuations of the any larger size species in the sample. This may be particularly
scattered light (detected at a backscattering angle of 173°) analyzed significant for aggregated or contaminated samples, but is also
to obtain an autocorrelation function. The software (DTS v5.03) of interest for broad, polydisperse non-homogeneous samples.
provided both the size mean and polydispersity, using the cumulants As a comparison, the polystyrene beads used for size standards
analysis (according to the international standard ISO 13321:19963) have typical polydispersity index values below 0.05. In order to
and a size distribution using a regularization scheme by intensity, understand the sample more completely it can be instructive to
volume, and number. look at the details of the size distribution, to indicate whether a
The following assumptions were made in the analysis: the solution non-homogeneous sample is a single broad mode or a number
viscosity was assumed to be that of water, corrected for temperature of modes. The direct output is a distribution by intensity, that is
(η = 0.888 mPa.s); the solution refractive index was that of the area of each of the modes is related to the intensity of light
water (n = 1.33); the refractive index of the particle was selected to scattered. Mathematically, the intensity distribution may be
be nR = 2.4 with an absorption of nI = 0.01. Samples were measured rescaled using Mie4 theory to transform the result into a volume
in disposable polystyrene cuvettes at a temperature of 25°C, and and a number distribution.
this temperature was actively maintained within 0.1°C in the sample
chamber. Data were acquired in automatic mode, ensuring enough Intensity distribution
photons were accumulated for the result to be statistically relevant. Figure 1 displays the intensity distribution produced by a typical
The software incorporated a ‘data quality report’ that indicated good algorithm. Here, the contributions in terms of % intensity are shown
quality for all data obtained. as a function of particle diameter. For an ideal monodisperse sample,
the peak in the intensity distribution is expected to be very close
Discussion to the z-average size from the cumulant fit. Here, we see a different
The sample gave an overall z-average size of 76.9 nm with a situation. As a consequence of the polydispersity of the sample,
polydispersity index of 0.10 indicating that the distribution the mean size of the peak by intensity is slightly different from the
consisted of a single size mode without aggregates. The result cumulant size. The mean of three repeat measurements reveals an
is an intensity-weighed value and will therefore be sensitive to intensity peak mean value of 85.6 nm.
MAY 2009 | VOLUME 12 | NUMBER 5 53
APPLICATIONS Light scattering and nanoparticles
Size Distribution by Number
30
Number (%)
20
10
0
0.1 1 10 100 1000 10000
Size (nm)
Record 47: TiO2 Nanotek, pH3, 1 Record 48: TiO2 Nanotek, pH3, 2
Record 49: TiO2 Nanotek, pH3, 3
Fig. 2 The number size distribution of Nanotek TiO2 versus particle diameter in nanometers is characterized by a peak mean of 51.9 nm. The different colors represent
repeat measurements of the same sample.
Volume and number distributions size. Again, Mie theory is used to perform the rescaling, and thus
Using Mie theory it is possible to convert the size distribution by the correct material properties (refractive index and absorption) are
intensity into a size distribution by mass or number. Mie theory needed for accurate results. As displayed in figure 2 the mean size is
requires information about the optical properties of the dispersing significantly smaller, in this case 51.9 nm. Number based metrologies
medium and the dispersed phase (both the real and imaginary part are typically microscopy techniques, where particle images are
of the refractive index) sometimes called the absorption, to do this counted and sorted into size bins. Again, when comparing size
transformation. The effect is that for particles below about 100 nm, results obtained with, for example electron microscopy, it must be
the volume and number means are smaller than the intensity mean. noted that the corresponding size distribution for comparison is the
Above this size, the change in the mean size reported will depend on number distribution.
the relative proportions of the different sizes in the distribution. This
is demonstrated in this case where the volume mean for the Nanotek Conclusions
TiO2 is 65.6 nm. When this light scattering result is compared with Using a sample of TiO2 we showed the difference between intensity,
other volume based detection techniques (for example ultraviolet volume, and number distributions. Using the same material, the
UV or refractive index RI detection in conjunction with size exclusion size given by an intensity based technique may differ by a factor
chromatography) it is important to use the volume size distribution two when compared with a number based technique. Neither of
for the comparison. these results is inherently the ‘right’ or ’correct’, but they indicate
In a similar way to the transformation from intensity to volume, why it is important to be clear about the technique being used
it is possible to calculate the result as a number distribution. Here, and the actual size parameter that is reported when referring to ‘a’
the number of particles is displayed as a function of the particle particle size.
REFERENCES 3. ISO13321:1996 Particle size analysis – Photon correlation spectroscopy,
1. Morfesis, A., and Fairhurst, D., Nanotech (2005) 1, 800. International Organization for Standardization (1996).
2. Chouly, C., et al., J. Microencapsul. (1996) 13(3), 245. 4. Mie, G., Annalen der Physik (1908) 330(3), 377.
54 MAY 2009 | VOLUME 12 | NUMBER 5
You might also like
- Bixby MaxFire and UBB Service ManualDocument71 pagesBixby MaxFire and UBB Service ManualAl Malley92% (13)
- Part B - 16 Mark QuestionsDocument5 pagesPart B - 16 Mark QuestionssubramanikcemechNo ratings yet
- Absorption of Gamma RaysDocument9 pagesAbsorption of Gamma RaysRachit KanchanNo ratings yet
- 1 6L GM Vortec SRVCDocument320 pages1 6L GM Vortec SRVCFred100% (2)
- Bird On Fire Lessons From The World's Least Sustainable CityDocument310 pagesBird On Fire Lessons From The World's Least Sustainable CityDavidNo ratings yet
- Kaszuba2008 Article MeasuringSubNanometreSizesUsin PDFDocument7 pagesKaszuba2008 Article MeasuringSubNanometreSizesUsin PDFJavier carretero mendozaNo ratings yet
- How To Measure Molecular Weight (Same Methode, Other Paper)Document6 pagesHow To Measure Molecular Weight (Same Methode, Other Paper)Julien HalnautNo ratings yet
- DTS Nano Series Training Course Size DayDocument98 pagesDTS Nano Series Training Course Size DayDerya KöseNo ratings yet
- Assets TN101104IntensityVolumeNumber 6 Tcm54 36773Document6 pagesAssets TN101104IntensityVolumeNumber 6 Tcm54 36773windli2012No ratings yet
- TXRF Application Note XRF 438 Ultratrace Element Analysis of Nanoparticles EN BRUKERDocument2 pagesTXRF Application Note XRF 438 Ultratrace Element Analysis of Nanoparticles EN BRUKERArif SumonNo ratings yet
- Panov 2018 J. Phys. Conf. Ser. 1092 012110 PDFDocument4 pagesPanov 2018 J. Phys. Conf. Ser. 1092 012110 PDFgerntrash2No ratings yet
- Resonance-Driven Random LasingDocument4 pagesResonance-Driven Random Lasingac10747No ratings yet
- Turbidimetric Determination of Particle Size Distributions of Colloidal SystemsDocument20 pagesTurbidimetric Determination of Particle Size Distributions of Colloidal SystemsailimillahNo ratings yet
- DLS - Basic Theory & ApplicationDocument6 pagesDLS - Basic Theory & ApplicationFábio Pohhi KrahôNo ratings yet
- Analysis of Optical Spectrum For Hematite Nanofluid Longpass Tunable FilterDocument5 pagesAnalysis of Optical Spectrum For Hematite Nanofluid Longpass Tunable FilterAJER JOURNALNo ratings yet
- MRK656-01 DLS in 30 MinutesDocument8 pagesMRK656-01 DLS in 30 MinutesQilu ZhangNo ratings yet
- Final ProjectDocument17 pagesFinal ProjectDhiraj KolheNo ratings yet
- Effect of 1-Thioglycerol As Capping Agent On ZNS Nanoparticles: Structural and Optical CharacterizationDocument4 pagesEffect of 1-Thioglycerol As Capping Agent On ZNS Nanoparticles: Structural and Optical CharacterizationInternational Journal of Science and Engineering InvestigationsNo ratings yet
- Abbe Refractometer: 1 ObjectiveDocument9 pagesAbbe Refractometer: 1 ObjectiveMuhammad AliNo ratings yet
- Far-Field Plasmonic Resonance Enhanced Nano-Particle Image Velocimetry Within A Micro ChannelDocument16 pagesFar-Field Plasmonic Resonance Enhanced Nano-Particle Image Velocimetry Within A Micro ChannelANo ratings yet
- T Richet 2016Document6 pagesT Richet 2016Johan Sebastian BuriticaNo ratings yet
- Superhelical Dna Studied by Solution Scattering and Computer ModelsDocument13 pagesSuperhelical Dna Studied by Solution Scattering and Computer ModelsDopameNo ratings yet
- 4 IJCMP JAN 2019 4 AnexactDocument3 pages4 IJCMP JAN 2019 4 AnexactErikNo ratings yet
- 5.isca RJRS 2012 356Document4 pages5.isca RJRS 2012 356prakush01975225403No ratings yet
- Ostwald Ripening of - Carotene NanoparticlesDocument4 pagesOstwald Ripening of - Carotene NanoparticlesEva Pa'e ONo ratings yet
- Size Analysis of Solid Particles Using Laser Diffraction and Sieve AnalysisDocument10 pagesSize Analysis of Solid Particles Using Laser Diffraction and Sieve AnalysisTatiana Monroy MoraNo ratings yet
- Estimating The Pitch Period of Voiced Speech: Indexing Terms: Speech Processing, MicroprocessorsDocument3 pagesEstimating The Pitch Period of Voiced Speech: Indexing Terms: Speech Processing, Microprocessorsasdf344No ratings yet
- Short Communication: Molecular Volumes and Densities of Liquids and Solids by Molecular Mechanics - Estimation AnalysisDocument6 pagesShort Communication: Molecular Volumes and Densities of Liquids and Solids by Molecular Mechanics - Estimation AnalysissemabayNo ratings yet
- Plugin Mie Theory The First Hundred Years MRK1304!02!1Document10 pagesPlugin Mie Theory The First Hundred Years MRK1304!02!1samrickyNo ratings yet
- EXPT. No. 1 Thickness Measurement of Plastic Foil Using Beta Back Scattering TechniqueDocument14 pagesEXPT. No. 1 Thickness Measurement of Plastic Foil Using Beta Back Scattering TechniqueAshish ManwarNo ratings yet
- NepheloturbidometryDocument3 pagesNepheloturbidometryNisha SharmaNo ratings yet
- Name of Student: Refractive IndexDocument4 pagesName of Student: Refractive IndexAddan JavidNo ratings yet
- Uv-Visible Spectroscopy - ManualDocument6 pagesUv-Visible Spectroscopy - ManualLikhithNo ratings yet
- Molecular Biophysics: Assignment 3Document9 pagesMolecular Biophysics: Assignment 3Kamal BoparaiNo ratings yet
- Comparing Experiment and Theory in Plasmonics: Home Search Collections Journals About Contact Us My IopscienceDocument10 pagesComparing Experiment and Theory in Plasmonics: Home Search Collections Journals About Contact Us My IopsciencetestNo ratings yet
- IG 1000 Article ICSDocument3 pagesIG 1000 Article ICSAshok KumarNo ratings yet
- Refractive Index ZnSe SellmeierDocument5 pagesRefractive Index ZnSe SellmeierDidier Alejandro Patiño RodriguezNo ratings yet
- P ViscoelasticPropertiesOfPolymerMeltsDocument4 pagesP ViscoelasticPropertiesOfPolymerMeltsSuchira SenNo ratings yet
- NepheloturbidimetryDocument18 pagesNepheloturbidimetrysushant.singhyadav19No ratings yet
- 3D Micro X-Ray Fluorescence Analysis: Wolfgang MalzerDocument8 pages3D Micro X-Ray Fluorescence Analysis: Wolfgang MalzeredsonmkNo ratings yet
- J 2011 Ultramicroscopy Psds Local ThresholdingDocument6 pagesJ 2011 Ultramicroscopy Psds Local Thresholdingwaseemkhan49No ratings yet
- Tracer Particles and Seeding For Particle Image Velocimetry: A MellingDocument11 pagesTracer Particles and Seeding For Particle Image Velocimetry: A MellingFSBollNo ratings yet
- (L10) Wave Optics PolarizationDocument44 pages(L10) Wave Optics PolarizationRahul Kumar SharmaNo ratings yet
- Lab Report 2 AOTWDocument14 pagesLab Report 2 AOTWFatihah AlhataNo ratings yet
- DLS Terms Defined MalvernDocument6 pagesDLS Terms Defined Malverncuongtran_siegenNo ratings yet
- Density Measurements: Rutherford Backscattering SpectrosDocument7 pagesDensity Measurements: Rutherford Backscattering SpectrosSalman Ali ShahNo ratings yet
- Refractive Index and Thickness Determination of Thin-Films Using Lloydõs InterferometerDocument8 pagesRefractive Index and Thickness Determination of Thin-Films Using Lloydõs InterferometerLuis MestaNo ratings yet
- New TechniquesDocument60 pagesNew Techniquesm a zargarNo ratings yet
- Revrew: Light-Scattering Immunoassay of Specific Proteins: ADocument14 pagesRevrew: Light-Scattering Immunoassay of Specific Proteins: AlaserstationNo ratings yet
- Synthesis of Zns / Sodium Hexameta Phosphate Nanoparticles: R. Mohan, B. Rajamannan and S. SankarrajanDocument6 pagesSynthesis of Zns / Sodium Hexameta Phosphate Nanoparticles: R. Mohan, B. Rajamannan and S. SankarrajanphysicsjournalNo ratings yet
- PIV Tracer-SeedingDocument12 pagesPIV Tracer-SeedingKaffelNo ratings yet
- Manual PH447Document96 pagesManual PH447swaroopNo ratings yet
- Newtons Rings Formal ReportDocument8 pagesNewtons Rings Formal ReportSammy BennettNo ratings yet
- Influence of Swelling of Noncrystalline Regions in Fibers On Modification With MethacrylamideDocument6 pagesInfluence of Swelling of Noncrystalline Regions in Fibers On Modification With Methacrylamideapi-3733260No ratings yet
- Tailoring The UV-visible Reflectivity Range of VO2 Thin FilmsDocument5 pagesTailoring The UV-visible Reflectivity Range of VO2 Thin FilmsDavid ArizaNo ratings yet
- Espectroscopia 3 inDocument19 pagesEspectroscopia 3 inErika ApazaNo ratings yet
- Simultaneous Control of Nanocrystal Size and Nanocrystal-Nanocrystal Separation in Cds Nanocrystal AssemblyDocument6 pagesSimultaneous Control of Nanocrystal Size and Nanocrystal-Nanocrystal Separation in Cds Nanocrystal Assemblyprakush01975225403No ratings yet
- Mat Cond 2010Document1 pageMat Cond 2010rubensufpaNo ratings yet
- Drop Size Distribution in Highly Concentrated Liquid-Liquid Dispersions Using A Light Back Scattering MethodDocument8 pagesDrop Size Distribution in Highly Concentrated Liquid-Liquid Dispersions Using A Light Back Scattering MethodDouglas SansaoNo ratings yet
- Goh 2014Document4 pagesGoh 2014syafira octaNo ratings yet
- Introduction To AgNPs-UBDocument14 pagesIntroduction To AgNPs-UBVaibhav NataNo ratings yet
- Colloidal Materials: Part IIIDocument22 pagesColloidal Materials: Part IIIUday Prakash SahuNo ratings yet
- Quantum Dots in A Polymer Composite: A Convenient Particle-in-a-Box Laboratory ExperimentDocument3 pagesQuantum Dots in A Polymer Composite: A Convenient Particle-in-a-Box Laboratory ExperimentRicardo ArnedoNo ratings yet
- Nuclear Techniques in Analytical Chemistry: International Series of Monographs on Analytical ChemistryFrom EverandNuclear Techniques in Analytical Chemistry: International Series of Monographs on Analytical ChemistryNo ratings yet
- Vantage BC Com v27 en 1108Document8 pagesVantage BC Com v27 en 1108Angel Quijano ArmengolNo ratings yet
- Vantage: Ultra-Low Maintenance BatteriesDocument8 pagesVantage: Ultra-Low Maintenance BatteriesAngel Quijano ArmengolNo ratings yet
- Single Cell RangeDocument27 pagesSingle Cell RangeAngel Quijano ArmengolNo ratings yet
- ALCAD L - M - H Ranges: Nickel Cadmium BatteriesDocument2 pagesALCAD L - M - H Ranges: Nickel Cadmium BatteriesAngel Quijano ArmengolNo ratings yet
- Vantage: Installation and Operating InstructionsDocument2 pagesVantage: Installation and Operating InstructionsAngel Quijano ArmengolNo ratings yet
- Instalacion y OperacionDocument2 pagesInstalacion y OperacionAngel Quijano ArmengolNo ratings yet
- Pro II 8.0 User GuideDocument390 pagesPro II 8.0 User GuideAngel Quijano ArmengolNo ratings yet
- Biophysical Chemistry: Diana Simionato, Stefania Basso, Giorgio M. Giacometti, Tomas MorosinottoDocument8 pagesBiophysical Chemistry: Diana Simionato, Stefania Basso, Giorgio M. Giacometti, Tomas MorosinottoAngel Quijano ArmengolNo ratings yet
- SUNON DC Brushless Fan & Blower (255-E)Document214 pagesSUNON DC Brushless Fan & Blower (255-E)Carlos X.No ratings yet
- Ansi-Hi Pump StandardsDocument2 pagesAnsi-Hi Pump StandardsAndrés Eduardo100% (1)
- Arc Welding: Assignment in Ce12Document5 pagesArc Welding: Assignment in Ce12Analyn NatividadNo ratings yet
- Advances in Carbon Nanotube N-Type Doping - Methods, Analysis and ApplicationsDocument37 pagesAdvances in Carbon Nanotube N-Type Doping - Methods, Analysis and Applicationslong rangeNo ratings yet
- SSR 2015-2016Document42 pagesSSR 2015-2016RamkumarNo ratings yet
- Aerzen Rental Covers Temporary Air Requirements: Dear ReadersDocument4 pagesAerzen Rental Covers Temporary Air Requirements: Dear ReadersFatih YAŞARNo ratings yet
- Living Eco VIIDB202Document28 pagesLiving Eco VIIDB202jovanoxNo ratings yet
- SPP Pumps - Tf15h - Dp6h-Ufaa50 - DrawingDocument1 pageSPP Pumps - Tf15h - Dp6h-Ufaa50 - Drawingroger cuipalNo ratings yet
- RobatechDocument8 pagesRobatechDavid OnceNo ratings yet
- Lecture 12Document43 pagesLecture 12salem bahsan100% (1)
- AUMA (Ba Sar2!07!16 Amb1 En)Document60 pagesAUMA (Ba Sar2!07!16 Amb1 En)cuongnammuNo ratings yet
- Nissan Almera 1995-2000 GIDocument48 pagesNissan Almera 1995-2000 GIHarePistaNo ratings yet
- Gunshot Effects SimulationDocument9 pagesGunshot Effects SimulationezedinNo ratings yet
- ABBDocument133 pagesABBIulian Luncan100% (3)
- STLE July 2018Document108 pagesSTLE July 2018Jorge Cuadros Blas100% (1)
- Simufact BR Rolling 2015 E Web PDFDocument2 pagesSimufact BR Rolling 2015 E Web PDFSamir KhNo ratings yet
- Chapter 1 Solutions Modern Physics 4th EditionDocument30 pagesChapter 1 Solutions Modern Physics 4th EditionDaniele CastroNo ratings yet
- Central Glass and Ceramic Research Institute - ProjectsDocument3 pagesCentral Glass and Ceramic Research Institute - ProjectsRavi Kant TripathiNo ratings yet
- BCT-UU 48V200Ah LiFePO4 Battery Pack 2021-9-2Document3 pagesBCT-UU 48V200Ah LiFePO4 Battery Pack 2021-9-2Abd AlhadiNo ratings yet
- Climate Change and Vulnerability in The Middle East - Carnegie Endowment For International PeaceDocument26 pagesClimate Change and Vulnerability in The Middle East - Carnegie Endowment For International PeaceEhzaz Ahmad ZahidNo ratings yet
- Financial Data & Ratio 2014 - SamplingDocument356 pagesFinancial Data & Ratio 2014 - SamplingeriwirandanaNo ratings yet
- Lyophilization of Parenterals FinalDocument25 pagesLyophilization of Parenterals FinalMostofa RubalNo ratings yet
- Shear Stress UU, CU, CDDocument101 pagesShear Stress UU, CU, CDMohamad Khaled NordinNo ratings yet
- Trident Tech LabDocument16 pagesTrident Tech LabkrcdewanewNo ratings yet
- 3.3 Busbar Protection SchemesDocument13 pages3.3 Busbar Protection SchemesAgu prasathNo ratings yet
- Axial Fans/Jet Fans: Installation and Operating Instructions GBDocument38 pagesAxial Fans/Jet Fans: Installation and Operating Instructions GBAleksandar PetrusevskiNo ratings yet