Professional Documents
Culture Documents
Constantes McWilliams PDF
Constantes McWilliams PDF
Uploaded by
Fernando0 ratings0% found this document useful (0 votes)
23 views1 pageOriginal Title
constantes-McWilliams.pdf
Copyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
23 views1 pageConstantes McWilliams PDF
Constantes McWilliams PDF
Uploaded by
FernandoCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You are on page 1of 1
Tante 2-3. Constants for fit to K values using Eq. (2-30)
‘Mean
Compound ay a a, Error
Methane — -292,860 0 59.8465 0 1.66
Ethylene 600.076.8750 7.90595 2.94594 0 2.65
Ethane 687,248.25 0 7.90694 49.02654 0 1.95
Propylene -923,484.6875 0 77S 41.67624 0 1.90
Propane -970,688.5625 0 7.15059 0 6.90224 2.35
Isobutane -1,166846 0 7.72668 -92213 0 0 252
n-Butane — -1,280,557 0 7.94986 -.96455 0 0 3.61
Isopentane -1,481,583 0 758071 93159 0 0 4.56
n-Pentane-1,524,891 0 7.33129 -.89143 0 0 4.30
n-Hexane -1,778,901 0 6.96783 -.84634 0 0 4.90
n-Heptane -2,013803 0 652914 -.79543 0 0 6.34
n-Octane 0 ~-1646.81641 1248457 -73152 0 0 7.58
n-Nonane 2,551,040 0 5.69313 67818 0 0 9.40
n-Decane 0 ~9760.45703 13.80354 71470 0 0 5.69
Note: isin °R, and pis in pia
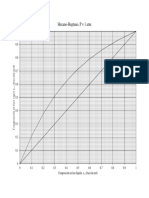
If only one component is present, then y = 1.0 and x= 1.0. This implies that K, = y/x = 10.
‘This gives a simple way of determining the boiling temperature of a pure compound at any
Pressure. For example, if we wish to find the boiling point of isobutane at p = 150 kPa, we
set our straightedge on p = 150 and at 1.0 on the isobutane scale on Figure 2-11. Then read
T= ~1.5°C as the boiling point. Alternatively, Eq. (2-30) with values from Table 2-3 can be
solved for T: This gives T = 488.68°R or -1.6°C.
For ideal systems Raoult’s law holds. Raoult’s law states that the partial pressure of a
component is equal to its vapor pressure multiplied by its mole fraction in the liquid. Thus,
Pa =Xq (VP)q (2-324)
where vapor pressure (VP) depends on temperature. By Dalton’s law of partial pressures,
Ya=PalP (2-32)
Combining these equations,
Ya = (VP) qxQ/P (2320)
Comparing Eqs. (2-32c) and (2-27), the Raoult’s law K value is
K,=(VP)y/p (2:33)
2.5 Multicomponent VLE
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5814)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1092)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (845)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (540)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (348)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (822)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Simulación de Procesos de SeparaciónDocument10 pagesSimulación de Procesos de SeparaciónFernandoNo ratings yet
- Rectificacion Por LotesDocument14 pagesRectificacion Por LotesFernandoNo ratings yet
- Practica 7 ElectroquimicaDocument15 pagesPractica 7 ElectroquimicaFernandoNo ratings yet
- Tecnicas Instrumentales AvanzadasDocument10 pagesTecnicas Instrumentales AvanzadasFernandoNo ratings yet
- C6-C7 MultietapasDocument2 pagesC6-C7 MultietapasFernandoNo ratings yet