Professional Documents
Culture Documents
Gland Corporate Brochure
Uploaded by
Anonymous ORleRr0 ratings0% found this document useful (0 votes)
4 views6 pagesdfdfdfdf
Copyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentdfdfdfdf
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
4 views6 pagesGland Corporate Brochure
Uploaded by
Anonymous ORleRrdfdfdfdf
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You are on page 1of 6
about
State-of-the-Art
World Class Manufacturing
Flagship Injectable Plant [iy
Dundigal Hyderabad
» Vials: 250 mil units/year
» Ampoules: 150 mil unitsiyear
» PES: 40 mil unitsiyear
» Lye Vials: 30 mil units/year
» Bags: 30 mil unitsiyear
» Ophthalmics: 60 mil units/year
12 Manufacturing Lines
» 6 Vial Lines with 8 Lyophilizers (1 - 100 m)
» 2 Ampaule Lines (1 - 10 m\)
» 2 PFS Lines (0.5 -6 mi)
» 1 Bag Filing Line (Fill volume 80 - 500 mi)
» 1 Ophthalmic Line
Penems Plant
Pashamiylaram, Hyderabad
Oncology Plant
Visakhapatnam
2 Manufacturing Lines » 1.4 mil unitsiyear
» Lyo Vials - 8 mie
» Lyo Vials - 5 miyear
» 2 Lines with 3 Lyophilzers (2 - 100 mi)
» Filling at 5°C temperature
» 1 Vial Line - Powder Filling (2-100 ml) » CRABS in Grade-B erea
» 1 Vial Line with 2 Lyophilzers (2-100 mi)
» Manufacture of products sensive
08S liners
Pashamylaram Plant
Pasharylaram, Hyderabad
» Vials: 200 milyear
» PFS: 100 miliear
» Lyo Vials: 50 milyear
» Ampoules: 100 milyear
8 Injectable Manufacturing Lines
» 2 Vial Lines (2 - 100 mi
» 2Vial Lines with
B Lyophilzers (2 - 100 mi
» 2PFS Lines (0.5 - 10 mi)
» 2 Ampaule Lines
Highlights
Leading Injectable manufacturer with in-house API development & manufacturing facilities
Danone va
Indian Company to set up a State-of-the-Art facility for Pre-filled Syringes (PFS)
SUC corel cc aa Waay()
Sao Seu ea UU ame a ar
Deets | OTT
Vertically integrated
One-Stop Shop
Active Pharmaceutical Ingredients - APIs
Development
» Synthetic Chemistry
» Analytical Chemisty
Manufacture
» API scale up
» Multiple Suites
n various Batch Sizes
» API facilities approved by EDOM & US FDA
Finished Product
Development
» Formulation development
* Analytical development
» Method development
» Packaging development
Manufacture
» Finished product manufacturing
» Quality Control & Quality Assurance
» Packaging
» Warehousing
» Shipping & Logistics
Quality Control &
Quality Assurance
© Quality Tec
OU et acur ke meric eta
+ Well-equipped in-house Quality Lab
asic ecm D\-\=1e) e008
Tone)
attest a acme ea?
eee aae mia aTeIAle]
+ Pilot and scale up stu
CELE)
* Formulation develo}
Peete ee ello
Or eroe eee Use a)
Pe)
OP Ne athe uae Ro
Sor Nee Camo Me Rance)
rimary reference standard preparation and
Services
Contract Manufacturing
de}
PANES
PaRORV Ely
Dr eet ec}
ANAL ter
> Ophthalmics
leone lesion ome eB oncne
» Synthetic Chemistry
> Formulation Development
» Analytical Methods and Validations
Pes eas eS
Out-licensing of Products
eee cen ence
Dea emcee cd
LacrelU LON
© Experienced in generic CTD/eCTD regulatory filings
Other Services
® Clinical Batch Manufacturing
“Global Generic Injectable Player
providing total solutions ”
Manufacturing “Facilities” approved by
FDA SaaS
MHRA - UK Tae cig
WHO - Geneva
TGA a ste Countries
BGV Reet Couey) a 3}
a - Czech Republic (EU) continents
ENVIS, aasicy
AN Seo 3
Sao BENT
NAFDAC SN orciey
NDA Uganda
PPB cone
&
GLAND PHARMA LIMITED
Regd, Office: 6-3-865/1/2 Greenland Apts., Ameerpet, Hyderabad - 500 016, India
Soe ta ea eee eee ere
Sree ae
You might also like
- Is Venous Congestion Associated With Reduced Cerebral Oxygenation and Worse Neurological Outcome After Cardiac Arrest?Document8 pagesIs Venous Congestion Associated With Reduced Cerebral Oxygenation and Worse Neurological Outcome After Cardiac Arrest?Anonymous ORleRrNo ratings yet
- Brain Sciences: Thiamine Deficiency Causes Long-Lasting Neurobehavioral Deficits in MiceDocument15 pagesBrain Sciences: Thiamine Deficiency Causes Long-Lasting Neurobehavioral Deficits in MiceAnonymous ORleRrNo ratings yet
- Basics of Mechanical VentilationDocument208 pagesBasics of Mechanical VentilationAnonymous ORleRr100% (1)
- Epidural Bupivacaine-Fentanyl Provides Superior Postoperative Pain Relief vs. IV PCA Morphine After Gynecological SurgeryDocument6 pagesEpidural Bupivacaine-Fentanyl Provides Superior Postoperative Pain Relief vs. IV PCA Morphine After Gynecological SurgeryAnonymous ORleRrNo ratings yet
- The Dog As An Animal Model For Intervertebral Disc Degeneration?Document9 pagesThe Dog As An Animal Model For Intervertebral Disc Degeneration?Anonymous ORleRrNo ratings yet
- OA 737-11-18 Comparison Between Effectiveness of Transtracheal Block Alone Versus Superior Laryngeal1Document5 pagesOA 737-11-18 Comparison Between Effectiveness of Transtracheal Block Alone Versus Superior Laryngeal1Anonymous ORleRrNo ratings yet
- OK Sio 2017Document6 pagesOK Sio 2017Anonymous ORleRrNo ratings yet
- Felsenstein 2020Document13 pagesFelsenstein 2020Mochamad RizkiNo ratings yet
- Prokalsitonin Dan Kultur Darah Sebagai Penanda Sepsis Di Rsup DR Wahidin Sudirohusodo MakassarDocument11 pagesProkalsitonin Dan Kultur Darah Sebagai Penanda Sepsis Di Rsup DR Wahidin Sudirohusodo MakassarAnonymous ORleRrNo ratings yet
- Cover Dalam Dan Abstrak Siti Aisya Sakinah Web PDFDocument3 pagesCover Dalam Dan Abstrak Siti Aisya Sakinah Web PDFAnonymous ORleRrNo ratings yet
- Acute Postoperative Pain at Rest After Hip and Knee Arthroplasty: Severity, Sensory Qualities and Impact On SleepDocument6 pagesAcute Postoperative Pain at Rest After Hip and Knee Arthroplasty: Severity, Sensory Qualities and Impact On SleepAnonymous ORleRrNo ratings yet
- Trends in Anaesthesia and Critical Care: Jamie Sleigh, Martyn Harvey, Logan Voss, Bill DennyDocument6 pagesTrends in Anaesthesia and Critical Care: Jamie Sleigh, Martyn Harvey, Logan Voss, Bill DennyAnonymous ORleRrNo ratings yet
- Management of Anembryonic Pregnancy Loss: An Observational StudyDocument6 pagesManagement of Anembryonic Pregnancy Loss: An Observational StudyAnonymous ORleRrNo ratings yet
- International Journal of Infectious Diseases: SciencedirectDocument4 pagesInternational Journal of Infectious Diseases: SciencedirectAnonymous ORleRrNo ratings yet
- Back Facts 10102Document2 pagesBack Facts 10102Anonymous ORleRrNo ratings yet
- Fluid Challenge Algorithm - 2006Document1 pageFluid Challenge Algorithm - 2006Anonymous ORleRrNo ratings yet
- ID Hubungan Faktor Resiko Dengan Terjadinya Nyeri Punggung Bawah Low Back Pain Pada PDFDocument10 pagesID Hubungan Faktor Resiko Dengan Terjadinya Nyeri Punggung Bawah Low Back Pain Pada PDFAnonymous aH8gCZ7zjNo ratings yet
- Platelets and Atherosclerosis: John C. Hoak, M.DDocument4 pagesPlatelets and Atherosclerosis: John C. Hoak, M.DAnonymous ORleRrNo ratings yet
- Diabetes & Metabolic Syndrome: Clinical Research & ReviewsDocument3 pagesDiabetes & Metabolic Syndrome: Clinical Research & ReviewsAnonymous ORleRrNo ratings yet
- Annals of EpidemiologyDocument7 pagesAnnals of EpidemiologyAnonymous ORleRrNo ratings yet
- Algo ArrestDocument2 pagesAlgo ArrestLocomotorica FK UkiNo ratings yet
- BJH 14482Document12 pagesBJH 14482Anonymous ORleRrNo ratings yet
- UNAIR, ASAD, Surabaya Sanglah Hospital, Bali BNDCC, Bali BNDCC, BaliDocument1 pageUNAIR, ASAD, Surabaya Sanglah Hospital, Bali BNDCC, Bali BNDCC, BaliAnonymous ORleRrNo ratings yet
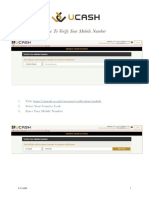
- How To Verify Your Mobile NumberDocument2 pagesHow To Verify Your Mobile NumberAnonymous ORleRrNo ratings yet
- BJH 14482Document12 pagesBJH 14482Anonymous ORleRrNo ratings yet
- 605 FullDocument3 pages605 FullAnonymous ORleRrNo ratings yet
- Nautic Us Privacy PolicyDocument3 pagesNautic Us Privacy PolicyAnonymous ORleRrNo ratings yet
- Perioperative Management of Thyroid DysfunctionDocument5 pagesPerioperative Management of Thyroid DysfunctionAnonymous ORleRrNo ratings yet
- Science of The Total Environment: Contents Lists Available atDocument8 pagesScience of The Total Environment: Contents Lists Available atAnonymous ORleRrNo ratings yet
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)