Professional Documents
Culture Documents
Late-Onset Pompe Disease With Left-Sided Bronchomalacia
Uploaded by
AnaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Late-Onset Pompe Disease With Left-Sided Bronchomalacia
Uploaded by
AnaCopyright:
Available Formats
Late-Onset Pompe Disease With Left-Sided Bronchomalacia
Chia-Feng Yang MD, Dau-Ming Niu MD PhD, Mei-Jy Jeng MD PhD,
Yu-Sheng Lee MD, Pei-Chen Taso MD, and Wen-Jue Soong MD
Pompe disease is a rare autosomal recessive disorder caused by ␣-glucosidase deficiency. Lower
airway involvement and management are rare in patients with late-onset Pompe disease. We
describe the case of a 16-y-old girl with late-onset Pompe disease who presented with obvious
progressive deterioration in respiratory function. Pulmonary hypertension was also apparent on
echocardiography. She had been on enzyme replacement therapy and nighttime CPAP ventilation
for several years. Flexible bronchoscopy was used for diagnosis and subsequent implantation of a
bronchial airway stent. Following implantation of the stent, the patient’s pulmonary function stabilized,
and her pulmonary hypertension resolved. The patient continued on enzyme replacement therapy and
nighttime CPAP ventilation. This case highlights that lower airway involvement may occur with late-
onset Pompe disease and that flexible bronchoscopy can be an effective tool for both diagnosis and
management of lower airway collapse in late-onset Pompe disease. Key words: bronchomalacia; bronchial
stent; flexible bronchoscopy; late-onset Pompe disease. [Respir Care 2015;60(2):e26 –e29. © 2015 Daedalus
Enterprises]
Introduction any age and is typically characterized by myopathy, respi-
ratory insufficiency, and obstructive sleep apnea.5-8 The
Pompe disease, also known as glycogen storage disease myocardium is usually not affected in late-onset Pompe
type II or acid maltase deficiency, is a very rare (1 in disease; however, cor pulmonale may occur. Regardless of
40,000 – 600,000) autosomal recessive disorder caused by the timing of onset, enzyme replacement therapy with re-
acid ␣-glucosidase deficiency and consequent accumula- combinant human acid ␣-glucosidase is recommended.1
tion of glycogen in lysosomes and cytoplasm.1,2 This ac- The upper airway, inspiratory muscles, and diaphragm
cumulation results in cell damage and has various clinical are frequently affected in late-onset Pompe disease,1
manifestations depending on the timing of onset. The most whereas, to the best of our knowledge, lower airway in-
severe form of Pompe disease is infantile-onset Pompe volvement has not been reported. Here, we present a unique
disease, which becomes apparent within the first 2 months case of late-onset Pompe disease with obvious left main
of life and is characterized by cardiac hypertrophy, hypo- bronchial malacia that was diagnosed and subsequently
tonia, respiratory distress, and delayed motor develop- managed with stent implantation by flexible bronchoscopy.
ment.3,4 Late-onset Pompe disease may become evident at
Case Report
The authors are affiliated with the Department of Pediatrics, Taipei Vet- A 16-y-old girl presented with respiratory distress in
erans General Hospital, Taipei, Taiwan. Drs Niu and Soong are also September 2012. She had previously been diagnosed with
affiliated with the Department of Pediatrics, School of Medicine, Na- late-onset Pompe disease (gene mutation c.2238G ⬎ C
tional Yang-Ming University, Taipei, Taiwan. [p.W746C]/c.2815_2816del [p.V939LfsX78]) at 9 y of age
The authors have disclosed no conflicts of interest. and had been receiving enzyme replacement therapy
(20 mg/kg every 2 weeks) since October 2007. Pulmonary
Correspondence: Wen-Jue Soong MD, National Yang-Ming University, function testing over the following years revealed restric-
No. 155, Section 2, Linong St, Taipei, 112 Taiwan, Republic of China.
E-mail: wjsoong@vghtpe.gov.tw. tive lung disease and progressively deteriorating lung func-
tion (Fig. 1); FVC and FEV1 had decreased by ⬎ 30% in
DOI: 10.4187/respcare.03419 a 3-y period. In 2009, the patient was diagnosed with
e26 RESPIRATORY CARE • FEBRUARY 2015 VOL 60 NO 2
POMPE DISEASE WITH BRONCHOMALACIA
Fig. 1. Summary of the patient’s pulmonary function parameters over an ⬃4-y period. PEF ⫽ peak expiratory flow.
Fig. 2. Flexible bronchoscopy images taken before (A) and after (B and C) implantation of a metallic mesh stent in the left main bronchial
lumen.
obstructive sleep apnea syndrome by polysomnography 137 g/L; and platelets, 180,000 cells/L. She was admitted
and was prescribed CPAP therapy. Overnight polysom- for respiratory examination and noninvasive ventilation.
nography performed in December 2011 revealed 59 hy- Flexible bronchoscopy under intravenous sedation revealed
popneic events and 2 apneic events. Echocardiography compromise of the pharynx, lower third tracheal malacia,
performed in July 2012 demonstrated: mild mitral valve and severe left main bronchial malacia with lumen col-
prolapse, thickening (0.38 cm), and regurgitation (grade lapse of ⬎ 90% (Fig. 2A). Chest computed tomography
2⫹); mild tricuspid valve prolapse and regurgitation (grade findings were consistent with flexible bronchoscopy find-
2⫹); and mild pulmonary hypertension (35 mm Hg), min- ings (Fig. 3). Due to the progressive dyspnea, she was
imal pulmonary regurgitation, and inferior vena cava dil- scheduled for aggressive management. A metallic expand-
atation without collapse during inspiration. able mesh stent (9 ⫻ 30 mm) was implanted smoothly
Upon presentation in our hospital, the girl reported a using a flexible bronchoscope (Figs. 2B and 4). After stent
recent history of sleep disturbance with difficult respira- implantation, the left main bronchial lumen became patent
tion. She also reported experiencing exercise dyspnea, chest (Fig. 2C) and clear at the first bifurcation. Enzyme re-
pain, and palpitations. She had mild scoliosis. Laboratory placement therapy and nighttime CPAP were continued.
results were as follows: creatinine, 0.33 mg/dL; lactate Follow-up echocardiography performed in August 2013
dehydrogenase, 311 U/L; alanine aminotransferase, 77 U/L; revealed no evidence of mitral valve prolapse or mitral or
aspartate aminotransferase, 109 U/L; creatine kinase, 305 pulmonary regurgitation and minimal tricuspid regurgita-
U/L; white blood cell count, 6,500 cells/L; hemoglobin, tion (grade 1⫺). Pulmonary pressure was 16 –20 mm Hg.
RESPIRATORY CARE • FEBRUARY 2015 VOL 60 NO 2 e27
POMPE DISEASE WITH BRONCHOMALACIA
(8 months after stent implantation), the left main bronchial
lumen and stent showed patency and no granulation for-
mation. The patient was attending school on a daily basis,
taking regular enzyme replacement therapy, and undergo-
ing follow-up examinations. Her pulmonary function was
stable, and the pulmonary hypertension had completely
resolved.
Discussion
To our knowledge, this is the first reported case of
late-onset Pompe disease with lower airway involvement.
In addition to the atypical involvement of the lower air-
way, the patient’s presentation was unusual in that she
exhibited precipitously declining lung function even with
ongoing enzyme replacement therapy, but did not appear
to have deteriorating skeletal muscle strength.
A previous study carried out in The Netherlands by van
der Beek et al9 showed that nearly three-quarters of sub-
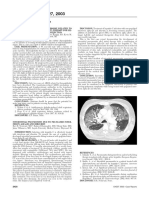
Fig. 3. Chest computed tomography image shows severe lumen jects with late-onset Pompe disease had pulmonary dys-
narrowing of left main bronchus (arrow).
function and that nearly 40% had clear respiratory muscle
weakness. Mild scoliosis was also quite common among
the 92 subjects included in the study. Disease duration,
skeletal muscle weakness, and sex were all found to be
predictors of pulmonary dysfunction, with males being
more severely affected than females. Interestingly, despite
the high level of respiratory involvement (nearly 30% re-
quired some form of respiratory support), a high propor-
tion (⬃40%) of the subjects had not undergone previous
vital capacity testing, emphasizing that respiratory testing
in patients with late-onset Pompe disease is underper-
formed. The study by van der Beek et al9 highlights the
key typical features of late-onset Pompe disease.
Clearly, any ongoing deterioration in respiratory func-
tion is concerning and necessitates the need for meticulous
examination and appropriate intervention(s) to reverse or
halt further progression. After admission, our patient un-
derwent flexible bronchoscopy examination, which re-
vealed not only upper airway compromise (common in
patients with Pompe disease) but also significant lower
airway narrowing with tracheomalacia and left main bron-
chial malacia. Flexible bronchoscopy was subsequently
Fig. 4. Chest radiograph after implantation of a left main bronchial safely used for both bronchial stent implantation and fol-
stent (arrow). low-up airway examinations. If flexible bronchoscopy is
used early, this management can detect not only respira-
tory lesions, if present, but also effectively halt further
Treadmill exercise testing revealed an improvement from deterioration and facilitate recovery from complications,
259 m (before stent) to 302 m in 6 min. Follow-up over- such as pulmonary hypertension and infection. This case
night polysomnography findings in August 2013 were con- leads us to suggest that a thorough airway examination is
sistent with a diagnosis of mild obstructive sleep apnea crucial for patients with late-onset Pompe disease who
syndrome (39 hypopneic events and 4 apneic events). No- experience an unexpectedly fast progression of respiratory
tably, CPAP completely ameliorated the obstructive sleep impairment. Indeed, regular flexible bronchoscopy exam-
apnea syndrome (one hypopneic event and zero apneic inations in these patients may be warranted to facilitate
events). At the last flexible bronchoscopy examination early recognition of any adverse changes.
e28 RESPIRATORY CARE • FEBRUARY 2015 VOL 60 NO 2
POMPE DISEASE WITH BRONCHOMALACIA
There are several potential mechanisms underlying the 3. van den Hout HM, Hop W, van Diggelen OP, Smeitink JA, Smit GP,
lower airway involvement in our patient with late-onset Poll-The BT, et al. The natural course of infantile Pompe’s disease:
20 original cases compared with 133 cases from the literature. Pe-
Pompe disease. Clearly, disease-related weakening of the
diatrics 2003;112(2):332-340.
posterior tracheobronchial muscles leading to dynamic col- 4. Kishnani PS, Hwu WL, Mandel H, Nicolino M, Yong F, Corzo D, et
lapse is likely. As noted, our patient had mild scoliosis, al. A retrospective, multinational, multicenter study on the natural
which may have contributed to thoracic insufficiency and history of infantile-onset Pompe disease. J Pediatr 2006;148(5):671-
hence respiratory insufficiency. General respiratory mus- 676.
cle involvement is also likely. 5. Müller-Felber W, Horvath R, Gempel K, Podskarbi T, Shin Y, Pon-
The case described herein highlights that lower airway gratz D, et al. Late onset Pompe disease: clinical and neurophysio-
logical spectrum of 38 patients including long-term follow-up in 18
compromise may occur with late-onset Pompe disease and
patients. Neuromuscul Disord 2007;17(9-10):698-706.
that flexible bronchoscopy can be an effective tool for 6. Wokke JH, Escolar DM, Pestronk A, Jaffe KM, Carter GT, van den
both diagnosis and management of airway manifestations Berg LH, et al. Clinical features of late-onset Pompe disease: a
of late-onset Pompe disease. As mentioned previously, prospective cohort study. Muscle Nerve 2008;38(4):1236-1245.
regular monitoring of respiratory function is important for 7. Chien YH, Lee NC, Huang HJ, Thurberg BL, Tsai FJ, Hwu WL.
patients with Pompe disease,9 regardless of enzyme re- Later-onset Pompe disease: early detection and early treatment ini-
placement therapy. Appropriate airway support should be tiation enabled by newborn screening. J Pediatr 2011;158(6):1023.e1-
1027.e1.
implemented as needed.
8. Chien YH, Lee NC, Huang PH, Lee WT, Thurberg BL, Hwu WL.
Early pathologic changes and responses to treatment in patients with
REFERENCES later-onset Pompe disease. Pediatr Neurol 2012;46(3):168-171.
1. Mellies U, Lofaso F. Pompe disease: a neuromuscular disease with 9. van der Beek NA, van Capelle CI, van der Velden-van Etten KI, Hop
respiratory muscle involvement. Respir Med 2009;103(4):477-484. WC, van den Berg B, Reuser AJ, et al. Rate of progression and
2. Pompe JC. [Concerning idiopathic hypertrophy of the heart]. Ned predictive factors for pulmonary outcome in children and adults with
Tijdschr Geneeskd 1932;76:304-311. Article in Dutch. Pompe disease. Mol Genet Metab 2011;104(1-2):129-136.
RESPIRATORY CARE • FEBRUARY 2015 VOL 60 NO 2 e29
You might also like
- Jurnal KoreaDocument6 pagesJurnal KoreaHerti PutriNo ratings yet
- Aydin Et Al 2011 A Case of Uncontrolled AsthmaDocument5 pagesAydin Et Al 2011 A Case of Uncontrolled AsthmadvbhodhanaNo ratings yet
- Use of Early Extra-Corporeal Membrane Oxygenation (ECMO) For Severe Refractory Status AsthmaticusDocument3 pagesUse of Early Extra-Corporeal Membrane Oxygenation (ECMO) For Severe Refractory Status Asthmaticusintanlidyawati26gmaiNo ratings yet
- Septic ShockDocument2 pagesSeptic ShockLatifah LàNo ratings yet
- Pediatric Round PneumoniaDocument4 pagesPediatric Round PneumoniaSalwa Zahra TsamaraNo ratings yet
- Bronchitis Obliterans Due To Mycoplasma PneumoniaDocument7 pagesBronchitis Obliterans Due To Mycoplasma Pneumoniawawa chenNo ratings yet
- Tension Pneumothorax in A Patient With COVID-19Document4 pagesTension Pneumothorax in A Patient With COVID-19Novie AstiniNo ratings yet
- A Case of Lupus Pneumonitis Mimicking As Infective PneumoniaDocument4 pagesA Case of Lupus Pneumonitis Mimicking As Infective PneumoniaIOSR Journal of PharmacyNo ratings yet
- Bilateral Massive Pulmonary Embolism Secondary To Decompression Sickness: A Case ReportDocument4 pagesBilateral Massive Pulmonary Embolism Secondary To Decompression Sickness: A Case ReportPatricio Toledo FuenzalidaNo ratings yet
- Sme PropofolDocument2 pagesSme PropofolAntonella Jazmín AssadNo ratings yet
- 415-Article Text-2894-1-10-20200228Document3 pages415-Article Text-2894-1-10-20200228Gianni CoccoNo ratings yet
- Unexpected Pulmonary Aspiration During Endoscopy Under Intravenous AnesthesiaDocument5 pagesUnexpected Pulmonary Aspiration During Endoscopy Under Intravenous AnesthesiaIta RositaNo ratings yet
- G. Hillerdal, J. Lee, A. Blomkvist, A. Rask-Andersen, M. Uddenfeldt, H. Koyi, E. RasmussenDocument5 pagesG. Hillerdal, J. Lee, A. Blomkvist, A. Rask-Andersen, M. Uddenfeldt, H. Koyi, E. Rasmussengarberer1No ratings yet
- Beluffi2009 Embolismo AereoDocument5 pagesBeluffi2009 Embolismo AereoPEDRO MUNYOZ ALVAREZNo ratings yet
- Eosinophilic Pneumonia-A Case ReportDocument1 pageEosinophilic Pneumonia-A Case ReportReitza RevilNo ratings yet
- Articulo. Neumomediastino EspontaneoDocument3 pagesArticulo. Neumomediastino EspontaneoEdRooNo ratings yet
- Epiglotitis DCHDocument6 pagesEpiglotitis DCHLu BfNo ratings yet
- ClassicsDocument2 pagesClassicsIndra Budi PermanaNo ratings yet
- Jurnal 7 BronkiolitisDocument7 pagesJurnal 7 BronkiolitisIrsanti sasmitaNo ratings yet
- Pulmonology Step2 CKDocument22 pagesPulmonology Step2 CKsarwat86% (7)
- Case Report (Septaecemia) : SummaryDocument3 pagesCase Report (Septaecemia) : Summaryikbal al-lamiNo ratings yet
- Cor Pulmonale Due To Obstructive Sleep Apnoea:, Case ReportDocument4 pagesCor Pulmonale Due To Obstructive Sleep Apnoea:, Case ReportMD MAHMUDUL HASAN NAEEMNo ratings yet
- Case Report Chronic CoughDocument2 pagesCase Report Chronic CoughVanetNo ratings yet
- Primary Malignant Melanoma of The TracheDocument93 pagesPrimary Malignant Melanoma of The TracheMikmik bay BayNo ratings yet
- RESP MEDICINE For PlabDocument73 pagesRESP MEDICINE For PlabJanie-Vi GorospeNo ratings yet
- Pneumothorax in Active Pulmonary Tuberculosis: Resurgence of An Old Complication?Document5 pagesPneumothorax in Active Pulmonary Tuberculosis: Resurgence of An Old Complication?M Tata SuhartaNo ratings yet
- Bronchoscopy AspergiloDocument5 pagesBronchoscopy AspergiloDevi EfrinaNo ratings yet
- MIEDEMA 2013 - Effect of Nasal Continuous and Biphasic Positive Airway Pressure On Lung Volume in Preterm InfantsDocument7 pagesMIEDEMA 2013 - Effect of Nasal Continuous and Biphasic Positive Airway Pressure On Lung Volume in Preterm InfantsRafael JustinoNo ratings yet
- Fungi52 52.9537Document6 pagesFungi52 52.9537Reza PutradinataNo ratings yet
- Anaesthesia Management in Endoscopic Ultrasound Guided Lung BiopsyDocument2 pagesAnaesthesia Management in Endoscopic Ultrasound Guided Lung BiopsyInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Bilateral Tension Pneumothorax Following Equipment ImprovisationDocument6 pagesBilateral Tension Pneumothorax Following Equipment ImprovisationIlvita MayasariNo ratings yet
- Airway-Centered Fibroelastosis 2016Document8 pagesAirway-Centered Fibroelastosis 2016Edoardo CavigliNo ratings yet
- Acute Pulmonary Edema Caused by Choking in An Adult PatientDocument5 pagesAcute Pulmonary Edema Caused by Choking in An Adult PatientTatas Bayu MursitoNo ratings yet
- Anestesia e Esclerose TuberosaDocument5 pagesAnestesia e Esclerose TuberosaMarina MelekNo ratings yet
- Bilat Ten Pneumo Improv 0214 p20 24Document5 pagesBilat Ten Pneumo Improv 0214 p20 24juliusNo ratings yet
- Helmet in VNIDocument9 pagesHelmet in VNIalexgonzalezherNo ratings yet
- 6 - WolfeDocument25 pages6 - WolfeEzeBorjesNo ratings yet
- NIH Public Access: Pulmonary Emphysema Subtypes On Computed Tomography in SmokersDocument23 pagesNIH Public Access: Pulmonary Emphysema Subtypes On Computed Tomography in SmokersNurulAzizahAnNo ratings yet
- Tracheal StenosisDocument4 pagesTracheal StenosisJessica MarianoNo ratings yet
- Medi 99 E19869 DikonversiDocument7 pagesMedi 99 E19869 DikonversiDr anshari NugrahaNo ratings yet
- CHEST Nov 2005Document183 pagesCHEST Nov 2005rizjkuramaNo ratings yet
- PEEP - Ventilação - Perfusão em Prona e Supino ArtigosDocument9 pagesPEEP - Ventilação - Perfusão em Prona e Supino Artigosle franchiNo ratings yet
- Eosinophilic Inflammation in Cough Variant Asthma: A. Niimi, R. Amitani, K. Suzuki, E. Tanaka, T. Murayama, F. KuzeDocument6 pagesEosinophilic Inflammation in Cough Variant Asthma: A. Niimi, R. Amitani, K. Suzuki, E. Tanaka, T. Murayama, F. KuzeYediftrisnawan 1995No ratings yet
- Treatment of COPD - The Sooner The BetterDocument5 pagesTreatment of COPD - The Sooner The BetterLorena ZirondiNo ratings yet
- Etm 13 5 2259 PDFDocument4 pagesEtm 13 5 2259 PDFdicky nanda muzidaNo ratings yet
- 498 FullDocument3 pages498 FullIndah D. RahmahNo ratings yet
- Hippokratia 13 187Document5 pagesHippokratia 13 187A.h.MuradNo ratings yet
- Ear Trauma Cpap Tinnitus jcsm.13.6.835Document2 pagesEar Trauma Cpap Tinnitus jcsm.13.6.835budNo ratings yet
- Accepted Manuscript: 10.1016/j.chest.2016.03.043Document22 pagesAccepted Manuscript: 10.1016/j.chest.2016.03.043Med Issam MahouachiNo ratings yet
- 500 FullDocument4 pages500 FullAina NurlailaNo ratings yet
- Articulo 7Document5 pagesArticulo 7Yuliana MoraNo ratings yet
- Antonelli - Fiberoptic Bronchoscopy During Noninvasive Positive Pressure Ventilation Delivered by HelmetDocument4 pagesAntonelli - Fiberoptic Bronchoscopy During Noninvasive Positive Pressure Ventilation Delivered by HelmetXaralyn XaviereNo ratings yet
- Borz One 2001Document6 pagesBorz One 2001Rodrigo Mañuico RicceNo ratings yet
- Infected Pulmonary Infarction Case ReportDocument8 pagesInfected Pulmonary Infarction Case ReportSebastian Felipe Sierra UmañaNo ratings yet
- Spontaneous Pneumomediastinum in Non-Asthmatic Children With Exercise-Induced BronchoconstrictionDocument4 pagesSpontaneous Pneumomediastinum in Non-Asthmatic Children With Exercise-Induced BronchoconstrictionprabuNo ratings yet
- MainDocument3 pagesMaindaeron42No ratings yet
- JTD 06 06 E77Document4 pagesJTD 06 06 E77jamieNo ratings yet
- Necrotizing Pneumonitis Caused by Mycoplasma Pneumoniae in Pediatric PatientsDocument4 pagesNecrotizing Pneumonitis Caused by Mycoplasma Pneumoniae in Pediatric Patientswawa chenNo ratings yet
- Interstitial Lung Disease: The Diagnostic Role of Bronchoscopy.Document15 pagesInterstitial Lung Disease: The Diagnostic Role of Bronchoscopy.Hitomi-No ratings yet
- Attachment PDFDocument2 pagesAttachment PDFYuyus Dwi PrasetiyoNo ratings yet
- Bronchoscopy in ICUDocument8 pagesBronchoscopy in ICUKumarasingham PirabaanandananNo ratings yet
- Kaposi SarcomaDocument34 pagesKaposi SarcomaDerri HafaNo ratings yet
- Quick Reference Handbook: Guidelines For Crises in AnaesthesiaDocument30 pagesQuick Reference Handbook: Guidelines For Crises in AnaesthesiaazeezNo ratings yet
- Near Misses in Pediatric AnesthesiaDocument253 pagesNear Misses in Pediatric Anesthesiashak31kem7420No ratings yet
- Surgical Instruments and Drains PDFDocument117 pagesSurgical Instruments and Drains PDFNariska CooperNo ratings yet
- NBIH Button Battery Ingestion Triage and Treatment GuidelineDocument6 pagesNBIH Button Battery Ingestion Triage and Treatment GuidelineWSETNo ratings yet
- EndoscopeOverview 2022 105x210 EN-GRZ 07 02 2022Document17 pagesEndoscopeOverview 2022 105x210 EN-GRZ 07 02 2022lied cnNo ratings yet
- Thomas J. Butler PH.D RRT RPFT - Laboratory Exercises For Competency in Respiratory Care-F.a. Davis Company (201Document865 pagesThomas J. Butler PH.D RRT RPFT - Laboratory Exercises For Competency in Respiratory Care-F.a. Davis Company (201Celia Seneh GwandiNo ratings yet
- Respi DX Procedures Handouts For Asynch ActivityDocument8 pagesRespi DX Procedures Handouts For Asynch ActivityBamba FloresNo ratings yet
- Paediatric Respiratory Reviews: Andreas P Eger, Ernst EberDocument9 pagesPaediatric Respiratory Reviews: Andreas P Eger, Ernst Eberadham bani younesNo ratings yet
- Congenital Laryngeal WebsDocument3 pagesCongenital Laryngeal WebsmitaNo ratings yet
- Competency Appraisal - Diagnostic TestsDocument7 pagesCompetency Appraisal - Diagnostic TestsMj BrionesNo ratings yet
- Medical Terminology EMED1201/ Mid-Term Exam PART I: Multiple Choice Questions - Select The One Best AnswerDocument7 pagesMedical Terminology EMED1201/ Mid-Term Exam PART I: Multiple Choice Questions - Select The One Best AnswerReham El-sawyNo ratings yet
- AFP Hemoptysis - 2022Document9 pagesAFP Hemoptysis - 2022Joshua DiaoNo ratings yet
- Foreign Body Ingestion and Aspiration in Dentistry: A Review of The Literature and Reports of Three CasesDocument8 pagesForeign Body Ingestion and Aspiration in Dentistry: A Review of The Literature and Reports of Three CasesgheaastridgayatriNo ratings yet
- All Notified Medical DevicesDocument228 pagesAll Notified Medical DevicesBALAJINo ratings yet
- OPA Mannequin Airways 2Document7 pagesOPA Mannequin Airways 2Betty BentonNo ratings yet
- Medind - Nic.in Iad t08 s1 Iadt08s1p688Document11 pagesMedind - Nic.in Iad t08 s1 Iadt08s1p688Anonymous S0H8cqgnfiNo ratings yet
- Nebulized Dexmedetomidine-Lidocaine Inhalation As A Premedication For Flexible Bronchoscopy: A Randomized TrialDocument8 pagesNebulized Dexmedetomidine-Lidocaine Inhalation As A Premedication For Flexible Bronchoscopy: A Randomized TrialRichard PhoNo ratings yet
- Ot - Role of Ot in Pulmonary RehabDocument32 pagesOt - Role of Ot in Pulmonary RehabAlyssa Bituin100% (1)
- Medically Compromised PatientsDocument21 pagesMedically Compromised PatientsDyah Wulan RamadhaniNo ratings yet
- Foreign Bodies in Aerodigestive Tract A Clinical Review of 109 Patients - July - 2020 - 6161115370 - 0822664Document3 pagesForeign Bodies in Aerodigestive Tract A Clinical Review of 109 Patients - July - 2020 - 6161115370 - 0822664Luis GallegosNo ratings yet
- ENT InstrumentsDocument9 pagesENT InstrumentsMuhammad Farrukh ul IslamNo ratings yet
- General Anesthesia For BronchosDocument7 pagesGeneral Anesthesia For BronchosMarites L. DomingoNo ratings yet
- Muhammad Rashid, Humaira Saleem, Saeed Bin Ayaz, Ijaz-Un-Nabi, Rashida Hussain, Faisal ManzoorDocument7 pagesMuhammad Rashid, Humaira Saleem, Saeed Bin Ayaz, Ijaz-Un-Nabi, Rashida Hussain, Faisal ManzoorEMIRZA NUR WICAKSONONo ratings yet
- Pediatrics StridorDocument13 pagesPediatrics StridorEmaNo ratings yet
- Antonelli - Fiberoptic Bronchoscopy During Noninvasive Positive Pressure Ventilation Delivered by HelmetDocument4 pagesAntonelli - Fiberoptic Bronchoscopy During Noninvasive Positive Pressure Ventilation Delivered by HelmetXaralyn XaviereNo ratings yet
- Aerodigestive Tract Foreign BodiesDocument53 pagesAerodigestive Tract Foreign BodiesAbhilash AntonyNo ratings yet