Professional Documents
Culture Documents
Lancet2019 PDF
Lancet2019 PDF
Uploaded by
danielOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Lancet2019 PDF
Lancet2019 PDF
Uploaded by
danielCopyright:
Available Formats
See discussions, stats, and author profiles for this publication at: https://www.researchgate.
net/publication/331310574
Lancet 2019
Article in The Lancet Infectious Diseases · February 2019
CITATIONS READS
0 816
6 authors, including:
Juan David Ramírez Hernan J Carrasco
Universidad del Rosario Instituto de Medicina Tropical, UCV
227 PUBLICATIONS 2,024 CITATIONS 181 PUBLICATIONS 1,813 CITATIONS
SEE PROFILE SEE PROFILE
Clara Elena Martinez Martin Llewellyn
Universidad Central de Venezuela, Caracas, Venezuela University of Glasgow
28 PUBLICATIONS 268 CITATIONS 149 PUBLICATIONS 3,040 CITATIONS
SEE PROFILE SEE PROFILE
Some of the authors of this publication are also working on these related projects:
Prevalence of Chagas disease in Venezuela and significant increase in risk factors for active transmission of the disease in endemic and no endemic regions View project
Leishmania donovani population structure View project
All content following this page was uploaded by Maikell Segovia on 24 February 2019.
The user has requested enhancement of the downloaded file.
Review
Venezuela’s humanitarian crisis, resurgence of vector-borne
diseases, and implications for spillover in the region
Maria E Grillet*, Juan V Hernández-Villena*, Martin S Llewellyn*, Alberto E Paniz-Mondolfi*, Adriana Tami*, Maria F Vincenti-Gonzalez,
Marilianna Marquez, Adriana C Mogollon-Mendoza, Carlos E Hernandez-Pereira, Juan D Plaza-Morr, Gabriella Blohm, Mario J Grijalva,
Jaime A Costales, Heather M Ferguson, Philipp Schwabl, Luis E Hernandez-Castro, Poppy H L Lamberton, Daniel G Streicker, Daniel T Haydon,
Michael A Miles, Alvaro Acosta-Serrano, Harry Acquattela, Maria G Basañez, Gustavo Benaim, Luis A Colmenares, Jan E Conn, Raul Espinoza,
Hector Freilij, Mary C Graterol-Gil, Peter J Hotez, Hirotomo Kato, John A Lednicky, Clara E Martinez, Santiago Mas-Coma, J Glen Morris Jr,
Juan C Navarro, Jose L Ramirez, Marlenes Rodriguez, Julio A Urbina, Leopoldo Villegas, Maikell J Segovia*, Hernan J Carrasco*, James L Crainey*,
Sergio L B Luz*, Juan D Moreno*, Oscar O Noya Gonzalez, Juan D Ramírez*, Belkisyolé Alarcón-de Noya*
In the past 5–10 years, Venezuela has faced a severe economic crisis, precipitated by political instability and declining Lancet Infect Dis 2019
oil revenue. Public health provision has been affected particularly. In this Review, we assess the impact of Venezuela’s Published Online
health-care crisis on vector-borne diseases, and the spillover into neighbouring countries. Between 2000 and 2015, February 21, 2019
http://dx.doi.org/10.1016/
Venezuela witnessed a 359% increase in malaria cases, followed by a 71% increase in 2017 (411 586 cases) compared
S1473-3099(18)30757-6
with 2016 (240 613). Neighbouring countries, such as Brazil, have reported an escalating trend of imported malaria
*Contributed equally
cases from Venezuela, from 1538 in 2014 to 3129 in 2017. In Venezuela, active Chagas disease transmission has been
Instituto de Zoología y
reported, with seroprevalence in children (<10 years), estimated to be as high as 12·5% in one community tested Ecología Tropical
(n=64). Dengue incidence increased by more than four times between 1990 and 2016. The estimated incidence of (Prof M E Grillet PhD,
chikungunya during its epidemic peak is 6975 cases per 100 000 people and that of Zika virus is 2057 cases per J V Hernández-Villena BSc),
100 000 people. The re-emergence of many vector-borne diseases represents a public health crisis in Venezuela and Instituto de Biología
Experimental
has the possibility of severely undermining regional disease elimination efforts. National, regional, and global (Prof G Benaim PhD), and
authorities must take action to address these worsening epidemics and prevent their expansion beyond Venezuelan Instituto de Medicina Tropical
borders. (M C Graterol-Gil MD,
C E Martinez PhD,
M Rodriguez MLT,
Introduction periodicity until November, 2014, when publication was M J Segovia MSc,
Over the past two decades, Venezuela has transitioned stopped by the government; the reports have not been Prof H J Carrasco PhD,
into a deep socioeconomic and political crisis. Once published since.8 In June, 2018, the Venezuelan Center L A Colmenares MD,
Prof O O Noya Gonzalez MD,
recognised as a regional leader for public health and for Classification of Diseases—a part of the Division of
Prof B Alarcón-de Noya MD)
vector-control policies and programming, Venezuela’s Epidemiology and Vital Statistics of the Ministry of Universidad Central de
health care has collapsed, creating a severe and ongoing Health that was in charge of providing the Pan American Venezuela, Caracas, Venezuela;
humanitarian crisis with no end in sight.1,2 It is a well Health Organization (PAHO) and WHO with updated Institute of Biodiversity,
Animal Health and
known fact that political and civil unrest can create morbidity and mortality indicators—was eliminated by
Comparative Medicine,
conditions for the emergence and spread of infectious the government after 63 years of uninterrupted activity.9 University of Glasgow,
diseases.3 Venezuela is no exception. With a decaying The return of measles and other vaccine-preventable Glasgow, UK (M S Llewellyn PhD,
health-care infrastructure, a mass departure of trained childhood infections in Venezuela since circa 2015 has Prof H M Ferguson PhD,
P Schwabl MSc,
medical personnel (a full medical professor earns been highlighted by PAHO.10 In this Review, we provide a
P H L Lamberton PhD,
<US$10 a month), and the decline of all public health comprehensive overview of the growing epidemics of the D G Streicker PhD,
programmes, the country is experiencing a surge and major vector-borne diseases—malaria, Chagas disease, Prof D T Haydon PhD);
expansion of vector-borne diseases. The UN High leishmaniasis, and arboviral infections—in Venezuela Infectious Diseases Research
Incubator and the Zoonosis
Commission for Refugees estimates that in 2014–18, and their ongoing spillover to neighbouring countries and Emerging Pathogens
1·5 million Venezuelans departed Venezuela for other based on the scarce data available. We examine the Regional Collaborative
countries in the Latin American and Caribbean region.4 potential effect of such spillover, and urge regional Network, Department of
By March, 2018, around 40 000 Venezuelans were health-care authorities to declare a public health Tropical Medicine and
Infectious Diseases, Instituto
estimated to be living in Brazil, and at least 600 000 people emergency of hemispheric concern. de Investigaciones Biomédicas
have sought shelter in Colombia.5,6 Official data are IDB, Clinica IDB Cabudare,
probably underestimates given the existence of informal Malaria: a regional menace Cabudare, Venezuela
border crossings. With these high amounts of emigration, Malaria, one of the most serious parasitic diseases of the (A E Paniz-Mondolfi MD,
M Marquez BSc,
reports of disease spillover to neighbouring countries are tropics, is caused by species of the genus Plasmodium A C Mogollon-Mendoza MD,
increasingly common.7 and transmitted among humans from the bites of C E Hernandez-Pereira BSc,
Disease surveillance and reporting have been equally infected female mosquitoes of the Anopheles genus. J D Plaza-Morr BSc,
affected by Venezuela’s health-care crisis. Since 1938, the WHO has established an ambitious plan for control and G Blohm PhD); Instituto de
Estudios Avanzados, Caracas,
Venezuelan Ministry of Health uninterruptedly issued elimination of the disease by 2030, and Latin American Venezuela (A E Paniz-Mondolfi,
weekly and monthly epidemiological reports known as countries have made substantial advances towards that Prof G Benaim); Department of
the Boletín Epidemiológico. However, in 2007, the reports goal, particularly from 2000 to 2015,11 when symptomatic Medical Microbiology,
had a 20-week interruption in publishing, regaining disease declined by 62% (from 1 181 095 cases in 2000 to University Medical Center
www.thelancet.com/infection Published online February 21, 2019 http://dx.doi.org/10.1016/S1473-3099(18)30757-6 1
Review
Groningen, University of 451 242 in 2015), and malaria-related deaths by 61·2% of miners and their families, combined with a deficit in
Groningen, Groningen, (from 410 in 2000 to 159 in 2015). Nonetheless, in 2016, a provision and implementation of curative and prevention
Netherlands (A Tami MD,
M F Vincenti-Gonzalez PhD);
considerable increase in case incidence (875 000) was services previously provided by the state, has created
Facultad de Ciencias de la reported in the region.12 Venezuela accounted for ideal conditions for malaria epidemics and increases in
Salud, Universidad de 34·4% of the total reported cases in 2016 (240 613). The morbidity and mortality. Since 2014, local malaria
Carabobo, Valencia, Venezuela number rose to 411 586 cases in 2017 (a 71% increase). transmission has re-emerged in new areas of the country,
(A Tami); Health Sciences
Department, College of
Incidence has been rising greatly since 2000 (increasing leading to a shift in the epidemiology of this disease.
Medicine, Universidad by 359% by 2015), particularly since 2010 (figure 1A). Endemic malaria transmission is now beginning to
Centrooccidental Lisandro During 2016, Plasmodium vivax accounted for 71% of propagate across the whole country, including urban and
Alvarado, Barquisimeto, Lara reported cases of malaria in Venezuela, followed by periurban foci, combined with an increase in hotspots,
State, Venezuela (M Marquez,
A C Mogollon-Mendoza,
Plasmodium falciparum (20%) and other plasmodium which persist in the Guayana Region (figure 1B).
C E Hernandez-Pereira); Health infections (approximately 9% of mixed and Plasmodium However, the numbers presented in figure 1B are likely
Sciences Department, College malariae cases).12 P vivax cases in Venezuela increased to represent an underestimate of the situation, as P vivax
of Medicine, Universidad from 62 850 in 2014, to 179 554 in 2016 (a three-fold case relapses are often not reported. Such cases are on
Nacional Experimental
Francisco de Miranda, Punto
increase). By the end of 2017, this number had increased the rise due to primaquine and chloroquine non-
Fijo, Falcón State, Venezuela by 76% to 316 401.12 Since 2017, numbers of mixed malaria adherence resulting from antimalarial drugs being out of
(J D Plaza-Morr); Emerging infections have increased, with double (P falciparum and stock. Further more, findings have also revealed that
Pathogens Institute,
P vivax) and triple (P vivax, P falciparum, and P malariae) there are four asymptomatic carriers per symptomatic
Department of Environmental
and Global Health, College of infections exhibiting rates higher than expected based case, with similar findings in Colombia and Brazil.20
Public Health and Health on usual occurrence for each species, reflecting high The rapidly increasing malaria burden in Venezuela
Professions, University of malaria transmission. Before 2003, malaria in Venezuela and the mass departure of its citizens continues to
Florida, Gainesville, FL, USA
followed an endemoepidemic pattern.12 Major incidence affect neighbouring countries, particularly Brazil and
(G Blohm, Prof J A Lednicky PhD,
Prof J G Morris Jr MD); Center for peaks occurred every 3–6 years in the two main ecological Colombia. According to the Brazilian Ministry of
Research on Health in Latin regions affected by malaria—namely, the southern Health, a total of 47 968 malaria cases were reported in
America, Escuela de Ciencias lowland rainforest and savannahs of the Guayana region, neighbouring Roraima from 2014 to 2017 (figure 2), of
Biológicas, Pontificia
and the wetlands of the northeastern coastal plains.13 which around 20% (9399) were imported from Venezuela.
Universidad Católica del
Ecuador, Quito, Ecuador From 2003 onwards, the Guayana region, particularly the Numbers of such cases increased from 1538 (in 2014) to
(J A Costales PhD); Infectious Sifontes Municipality in southeastern Bolívar State, 3129 (in 2017). In 2016, 45% of reported malaria cases in
and Tropical Disease Institute, Venezuela (figure 1B), became the highest risk area for the Brazilian border municipality of Pacaraima, and
Department of Biomedical
malaria in the country.13,14 In the Sifontes Municipality, 86% of cases in another border municipality, Boa Vista,
Sciences, Heritage College of
Osteopathic Medicine, Ohio malaria incidence is likely to be linked with an increase were attributed to Venezuelan immigration (figure 2C).
University, Athens, OH, USA in illegal mining activities and forest exploitation. A The continued upsurge of malaria in Venezuela could
(Prof M J Grijalva PhD); College complex pattern of localised, albeit persistent, hotspots soon become uncontrollable, jeopardising the hard-won
of Medical, Veterinary
and Life Sciences
of plasmodium transmission is maintained principally gains of the malaria control programme in Brazil and
(L E Hernandez-Castro MSc) and by Anopheles (soon to be Nyssorhynchus) darlingi Root other countries in the region. With 411 586 cases in 2017,
MRC-University of Glasgow (4·0%),15 Anopheles albitarsis Lynch sl (5·4%), and Venezuela might now exhibit the largest malaria
Centre for Virus Research, Anopheles nuneztovari Gabaldon sl (0·5%),16,17 which show increase reported worldwide,7 threatening the successful
University of Glasgow,
Glasgow, UK (D G Streicker);
high natural parasite infections. implementation of the WHO Global Technical Strategy
Department of Pathogen Clearing forests for mining activities has been observed for Malaria.7,12
Molecular Biology, London to create favourable conditions for A darlingi and
School of Hygiene & Tropical A albitarsis breeding.18 An increase in illegal mining Chagas disease: persistent endemism and
Medicine, London, UK
(Prof M A Miles PhD);
activities is likely to be linked to the economic crisis. resurgence
Department of Vector Biology Highly mobile, often immunologically naive, human Chagas disease is caused by the kinetoplastid parasite
and Department of populations migrate from different regions of the country Trypanosoma cruzi, which currently infects approximately
Parasitology, Liverpool School to mining areas in search of economic opportunities. 6 million people worldwide.21 Chagas disease is a complex
of Tropical Medicine, Liverpool,
UK (A Acosta-Serrano PhD,
Once people arrive, they live outdoors, constantly zoonosis involving multiple species of mammal and blood-
M G Basañez PhD); Venezuelan exposed to the risk of mosquito bites. Many internal sucking triatomine bug.22 In approximately 30–40% of
National Academy of Medicine, migrants return to previous endemic malaria regions cases, human infection with T cruzi leads to severe, and
Caracas, Venezuela where viable anopheles vector populations exist, irreversible, cardiac and intestinal pathology.21 Chagas
(Prof H Acquattela MD); Griffin
Laboratory, Wadsworth Center,
reintroducing malaria to areas where this infection had
New York State Department of been previously eliminated. Additionally, financial
Health, Albany, NY, USA constraints generated by the crisis in the past 5 years Figure 1: Annual parasite incidence and confirmed cases of
(J E Conn PhD); School of Public
have severely restricted the procurement of malaria malaria in Venezuela
Health, University at Albany,
commodities (eg, insecticides, drugs, diagnostic supplies, (A) Number of confirmed malaria cases (line) and annual parasite incidence per
NY, USA (J E Conn); Hospital
1000 inhabitants (bars) in Venezuela, 2000–16. Inset left: map of Venezuela
Miguel Pérez Carreño, Instituto and mosquito nets), and have hampered epidemiological
(red) in South America. Inset right: case comparison of annual parasite incidence
Venezolano de los Seguros surveillance, reporting activities, vector control, and for Colombia, Brazil, and Venezuela. (B) Annual parasite incidence for each
Sociales, Caracas, Venezuela
disease treatment efforts.2,19 Internal economic migration municipality in Venezuela during 2016. *Sifontes Municipality.
2 www.thelancet.com/infection Published online February 21, 2019 http://dx.doi.org/10.1016/S1473-3099(18)30757-6
Review
(Prof R Espinoza PhD); Hospital
de Niños Ricardo Gutiérrez,
Buenos Aires, Argentina
A (H Freilij MD); National School
250 000 of Tropical Medicine, Baylor
Brazil Colombia Venezuela 25
30 College of Medicine, Houston,
25 22·5 TX, USA (Prof P J Hotez MD);
Annual parasite incidence
(per 1000 inhabitants)
22·1
Division of Medical Zoology,
20
Department of Infection and
200 000 15 20 Immunity, Jichi Medical
Annual parasite incidence (per 1000 inhabitants)
University, Tochigi,
10
Japan (Prof H Kato PhD);
5 Departamento de
15·3 Parasitología, Universidad de
0
150 000 2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 13·5 15 Valencia, Valencia, Spain
Time (year) (Prof S Mas-Coma PhD);
Cases (n)
Enfermedades Emergentes y
Salud Ambiental, Centro de
9·3 Biodiversidad, Universidad
100 000 8·9 10
8·4 Internacional SEK, Quito,
8·0
Ecuador (Prof J C Navarro PhD);
6·1 Biotechnology Center,
5·6 5·8 5·4
5·0 5·2 Instituto de Estudios
50 000 5 Avanzados, Caracas, Venezuela
3·4 3·9
(Prof J L Ramirez PhD);
2·3
Venezuelan Institute for
Scientific Research, Caracas,
Venezuela (Prof J A Urbina PhD);
0 0 Asociación Civil Impacto Social,
2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017 Tumeremo, Bolívar State,
Time (year) Venezuela (L Villegas MD);
Instituto Leônidas e Maria
B Deane ILMD/FIOCRUZ,
Laboratório de Ecologia de
Doenças Transmissíveis na
Amazônia, Manaus, Amazonas,
Brazil (J L Crainey PhD,
S L B Luz PhD); Centro de
Investigaciones de Campo
“Dr Francesco Vitanza”, Servicio
Autónomo Instituto de Altos
Estudios “Dr Arnoldo
Gabaldon”, MPPS, Tumeremo,
Venezuela Venezuela (J D Moreno PhD,
Prof O O Noya Gonzalez); and
Grupo de Investigaciones
Microbiológicas-UR, Programa
de Biología, Universidad del
Rosario, Bogotá, Colombia
(J D Ramírez PhD)
Correspondence to:
Guyana Dr Martin Llewellyn, Institute of
Biodiversity, Animal Health and
Comparative Medicine,
University of Glasgow,
Glasgow, G12 8QQ, UK
martin.llewellyn@glasgow.ac.
uk
Annual parasite incidence
0
1–15
16–30
31–60
61–125
126–250
251–500
501–1000
1001–1640 0 80 160 320 400 km
www.thelancet.com/infection Published online February 21, 2019 http://dx.doi.org/10.1016/S1473-3099(18)30757-6 3
Review
A B
Guyana Guyana
Venezuela
Venezuela
Brazil Brazil
C
Origin of cases
Imported cases from Venezuela
Autochthonous cases *
Number of cases
0
Guyana 1–625
626–1250
Venezuela 1251–2500
2501–5000
5001–10 000
10 001–22 000 †
22 001–72 000
72 001–1102 550
Road
Brazil
0 60 120 180 360 540 km
Figure 2: Malaria cases reported in eastern Venezuela and neighbouring Roraima State, Brazil
(A) 2014, (B) 2015, and (C) 2016. Pie charts indicate origin of cases. Inset shows the locations of Pacaraima and Boa Vista. *Pacaraima. †Boa Vista.
disease has remained endemic in Venezuela since its first invades and colonises rural houses from wild transmission
description in 1919. In the 1960s and 1970s, seroprevalence cycles after insecticide spraying. Thus, even before the
was 43·9% overall, and 20·4% in young children current economic crisis, Venezuela was at risk of resurgent
(aged <10 years, a key indicator of active transmission).23,24 Chagas disease. Since 2012, the surveillance and control of
Efforts to interrupt Chagas disease transmission, alongside Chagas disease transmission in Venezuela have been
widespread insecticide use against malaria vectors, abandoned. In this Review, we can report the multiple
succeeded in reducing seroprevalance to 9·2% and the hotspots of new and active disease transmission by piecing
geographical extent of transmission risk by 52%.25 together unpublished data from several sources.
Seroprevalence among children younger than 10 years was In the Andes and western Venezuela, Chagas disease
reduced to 0·5% between 1990 and 1998.25 Regrettably, the is present throughout many different states. Sero-
1990s saw the national Chagas disease control programme prevalences obtained from three endemic communities
reduced and decentralised.25 Moreover, Chagas disease in Portuguesa State, Venezuela, between 2014 and 2016
See Online for appendix control in Venezuela has been hindered by the ecology of (12·5% in those <10 years; figure 3A, appendix) show
the principal vector, Rhodnius prolixus, which frequently considerable active transmission. Also, house infestation
4 www.thelancet.com/infection Published online February 21, 2019 http://dx.doi.org/10.1016/S1473-3099(18)30757-6
Review
Nueva Esparta
Sucre
Portuguesa
Anzoátegui
Guárico
0 60 120 130 260 390 km
Seroprevalence (%) Age classes (years)
ND 1–10 41–50
3 11–20 51–60
6·9 21–30 61–70
8·3 31–40 71–80
17·7 81–90
53·4 Oral outbreaks
B
Number of triatomines
0 251–300
1 301–350
2–50 351–400
51–100 401–500
101–150 501–600
151–200 >600
201–250
T cruzi infection prevalnce (%)
0 41–50
1–10 51–60
11–20 61–70
21–30 71–80
31–40 81–90
Figure 3: Chagas disease and Trypanosoma cruzi distribution in Venezuela
(A) Update on the distribution of Chagas disease human seroprevalance data and sites of oral outbreaks in Venezuela. States for which data are available are coloured by
percentage overall seroprevalence. Pie charts indicate infection among different age classes. Blue diamonds indicate sites of reported oral outbreaks. (B) Distribution of
peri-urban vectors and T cruzi infection status around Caracas. Upper map shows data for triatomines brought to the clinic at the Insituto de Medicine Tropical, Caracas,
Venezuela, in 2007–16 by municipality. Lower map shows T cruzi infection prevalence (%) in the same vectors. Inset shows locations of sampled neighbourhoods in
Venezuela. ND=no data available.
www.thelancet.com/infection Published online February 21, 2019 http://dx.doi.org/10.1016/S1473-3099(18)30757-6 5
Review
indices were estimated to be as high as 24·8% in some Half of these outbreaks have occurred in and around
hotspots at the time the Chagas disease control Caracas, although reports from other geographical
programme was dismantled.26 Seroprevalences observed regions are increasing, with many undiagnosed cases
in Lara State in 2011 (0·57% in those <10 years) also probably remaining unreported because of the non-
suggest some active transmission.26,27 Seroprevalence specific signs and symptoms as well as physicians’
estimates from 2011–19 for Lara State and other western unfamiliarity with the acute phase of the disease. Severe
states are not available; however, Chagas disease might drug shortages have forced patients to cross borders into
well be resurgent, since no surveillance or preventative neighbouring countries in search of treatment, medical
measures are in place. At the time of publishing, an care, or both. Moreover, the monitoring of these patients
outbreak of acute Chagas disease in Táchira State, is essential because treatment with the only existing
Venezuela, reported in the Colombian media, had drugs (benznidazole and nifurtimox) is not totally
infected 40 people and claimed eight lives.28 11 cases in effective, a situation exacerbated by the low availability of
total of so-called spillover acute disease in Venezuelan these drugs in Venezuela, and the medical personnel to
nationals were confirmed by the Colombian authorities administer them.34
between October, 2017 and April, 2018. By contrast with Linked to several oral Chagas disease outbreaks in and
western Venezuela, in the 2000s, studies suggested that around Caracas are increasing reports of peri-urban
elimination of vectorial transmission of Chagas disease transmission of T cruzi in Venezuela. The presence of
in eastern Venezuela was possible.25 However, sero- T cruzi was first reported in 2005, when 76·1% of the
prevalence data show that active transmission is present disease vector P geniculatus recovered from the capital
in Venezuela in Nueva Esparta State (2·5% of those aged district and the states of Miranda and Vargas were naturally
1–9 years in 2016; figure 3A, appendix), Anzoátegui State infected with T cruzi, and that 60·2% of their gut contents
(8% of those aged 11–20 years in 2014; figure 3A, gave a positive reaction to human antiserum.35 Ongoing
appendix), and Sucre State (2% of those aged 11–20 years collections between 2007 and 2016 have continued to
in 2012; appendix). In Nueva Esparta State, most reveal mostly P geniculatus (98·96%), as well as Triatoma
seropositive individuals were young or members of the nigromaculata (0·58%), Triatoma maculata (0·37%),
older population, possibly reflecting the success of the R prolixus (0·07%), and Panstrongylus rufutuberculatus
former control programme and current resurgence of (0·02%; figure 3B).36 Vector infection rates with T cruzi
the disease (figure 3A, appendix). Overall seroprevalence over this period have been consistently high (75·7%; figure
among children from the data reported in the appendix 3B). Intradomiciliary triatomine nymphs also present in
and figure 3A (4·3% of those <10 years) indicates 16 of the 32 parishes (3·42% of vectors captured) suggest
resurgent infection and resembles rate estimates from active colonisation of houses by these insects. Preliminary
the 1970s.25 However, our sample sizes are at least one molecular analysis of blood meals identify humans as by
order of magnitude lower than historical studies, far the most common blood-feeding source among
although the serological approaches were similar (ELISA, insects collected in 2007–16. Furthermore, molecular
indirect haemagglutination [IHA], and flow cytometry epidemiological analyses clearly identify parasites from
[FC]). Nationally, seroprevalences over all age groups these peri-urban transmission cycles as the source of local
(15·7%; appendix) exceed those in endemic provinces in oral outbreaks.37 To what extent vectors are also transmitting
Colombia (2·2% in Boyacá in 2007–09; 0·2% in parasites directly to human populations in the metropolitan
Santander in 2013–14)29 and Ecuador (3·5% in Manabí, district (ie, not orally via contaminated food) is not known.
Loja, Guayas in 2001–03)30 by a substantial margin. However, given the high amount of feeding on humans
Whether blood banks are still being screened for Chagas and the high number of infected triatomines, vectoral
disease in Venezuela is unclear; however, in the current transmission remains a possibility despite the supposed
crisis it seems unlikely. low vectoral capacity of P geniculatus.36
Oral Chagas disease transmission has also become
an issue of great concern. Between 2007 and 2018, Leishmaniasis: an early wake-up call
16 outbreaks of oral Chagas disease have been recorded Leishmaniasis refers to a spectrum of diseases caused by
nationwide, and ten have been managed through the several trypanosomatid species belonging to the genus
outpatient clinic of the Institute of Tropical Medicine, Leishmania (Old and New World) and subgenus Viania
Caracas, Venezuela; figure 3B, appendix).31,32 The updated (New World). Leishmania spp are transmitted through
data in the appendix show 321 cases and 23 deaths in the bite of infected phlebotomine sandflies. In Venezuela,
10 years. Such outbreaks have frequently been associated leishmaniasis is widely distributed, with most endemic
with consumption of artisan fruit juices contaminated zones located throughout the valleys of the coastal
with infected triatomines (especially the vector species mountain range, the Yaracuy River basin (west), some
Panstrongylus geniculatus) or their faeces, and show a areas of the central plains (Los Llanos), and the Andean
severe clinical course and high mortality.33 Urbanisation mountain forests. Isolated endemic foci south of the
and deforestation of wooded areas where the triatomines Orinoco River in the Amazon basin have also been
are present might also be contributing to this situation.2 reported, but remain to be fully characterised.38
6 www.thelancet.com/infection Published online February 21, 2019 http://dx.doi.org/10.1016/S1473-3099(18)30757-6
Review
As per data from the National Sanitary Dermatology A
Programme of the Venezuelan Minister of Health,39 3000 9·5 10
61 576 cases of cutaneous leishmaniasis occurred 9·0
8·6
Annual parasite incidence (per 1000 inhabitants)
9
between 1990 and 2016, with approximately 75% of the 8·0
2500
cases occurring in the Venezuelan states of Táchira, 7·0 7·1
8
6·6 6·6
Mérida, Trujillo, Lara, Miranda, and Sucre (figure 4). 7
2000
Since 2006, regions of the leishmaniasis endemic in 5·4 5·5
5·4
6
Venezuela have expanded substantially. This expansion
Cases (n)
1500 5
is probably linked to ever-increasing trends towards
urbanisation, deforestation, environmental changes, and 4
1000
the emergence of focal peri-urban transmission cycles as 3
reported in several cities in the states of Lara and
500 2
Trujillo.38 Nothing in the available data suggests that
the frequency of different clinical manifestations of 1
0 0
cutaneous leishmaniasis (mucocutaneous, disseminated,
06
08
09
16
14
07
10
12
13
15
11
and diffuse) has been affected by the crisis.
20
20
20
20
20
20
20
20
20
20
20
Visceral leishmaniasis is prevalent in three endemic Time (year)
foci across Venezuela. The central focal point includes B
the states of Guárico, Carabobo, Cojedes, and Aragua; the
Caracas
western focal point covers the states of Portuguesa, Lara, Amazonas
and Trujillo; and the eastern focal point, includes the 0 80 700 1500
Anzóategui
states of Sucre, Anzoátegui, and the insular state of Apure
Nueva Esparta.38 Between 1961 and 1991, reports revealed Aragua
the occurrence of 675 cases nationwide; however, this Barinas
Bolívar
might be an underestimate of the real situation. Infected
Carabobo
dogs can develop visceral leishmaniasis and are a
Cojedes
potential source of infection to both other dogs and Delta Amacuro
humans. Sero-epidemiological surveys indicate that Falcón
between 2004 and 2012, the prevalence of visceral Guárico
leishmaniasis was 14·8% among 15 822 dogs evaluated, Lara
with the states of Lara and Guárico showing the highest Mérida
Miranda
seroprevalence, and most dogs (81%) showing no clinical
Monagas
signs.40 Migratory trends might be contributing to the Potuguesa
spread of the disease from its traditional endemic rural Sucre
niches into peri-urban ecotopes where the presence of Táchira
vectors (Lutzomyia longipalpis and Lutzomyia evansi) Trujillo
could aid the instalment of new autochthonous foci.38 Vargas
Yaracuy
The risk of Leishmania spp transmission has historically
Zulia
been affected by migrations, refugee crises, wars, and
2006
2008
2009
2016
2014
2007
2010
2012
2015
2013
2011
states of civil unrest, which can encourage cross-border
movement of cases—notably observed during conflicts
in Syria and Yemen since 2010.32–37,41–43 Cross-border Figure 4: Annual parasite incidence and confirmed cases of cutaneous leishmaniasis in Venezuela
(A) Number of confirmed cases (line) and annual incidence per 100 000 inhabitants (bars) in Venezuela, 2006–16.
dispersal of Leishmania spp from Venezuela is already (B) Annual parasite incidence by state per 100 000 inhabitants for 2006–16 (increasing from blue to red).
occurring, and several cases of visceral leishmaniasis
and cutaneous leishmaniasis have been detected in
Venezuelan migrants to Colombia in the past 6 months.44 previously unaffected areas. All three arboviruses are
transmitted by the same mosquito, Aedes aegypti (L), with
Arboviruses: an expanding threat a potential role for the invasive species Aedes albopictus
Viruses that are transmitted by arthropod vectors as well.
(arboviruses) have been expanding either steadily or in A member of the Flaviviridae family, dengue virus has
explosive emergent or re-emergent epidemics over the become a major public health problem in Venezuela.
past 10 years, posing a growing threat to global public Four dengue virus serotypes (DENV-1–4) co-circulate in
health.45,46 In the past 4 years, the two major epidemics the country, each of them capable of causing the entire
that swept the American continent were caused by the range of dengue-related disease symptoms. Infected
chikungunya and Zika arboviruses.47 Simultaneously, individuals can be asymptomatic or present with clinical
dengue virus, another arboviral disease endemic in the manifestations varying from mild febrile illness, to severe
Latin American region, is increasing its spread to disease and death.48 Venezuela is witnessing an upswing
www.thelancet.com/infection Published online February 21, 2019 http://dx.doi.org/10.1016/S1473-3099(18)30757-6 7
Review
(per 100 000 inhabitants)
1000 Brazil Colombia Venezuela
Annual incidence
500 800
600
450
400
Annual incidence (per 100 000 inhabitants)
400 200
350 0
14
10
12
13
09
11
15
08
07
06
16
20
20
20
20
20
300
20
20
20
20
20
20
Time (year)
250
200
250
100
50
0
90
91
92
93
94
95
96
97
98
99
00
01
02
03
04
05
06
07
08
09
10
11
12
13
14
15
16
17
20
20
20
20
20
20
20
20
20
19
20
19
20
20
20
19
19
19
20
20
19
20
20
20
19
19
19
19
Time (years)
B
Falcón
Yaracuy
Carabobo*
0 5 15 35 50
Aragua*
Caracas*
Miranda*
Vargas
Anzóategui
Monagas
Sucre
Nueva Esparta
Delta Amacuro
Trujillo
Lara
Potuguesa
Cojedes
Guárico
Mérida
Barinas
Zulia†
Táchira†
Apure†
Amazonas†
Bolívar†
1996
1998
1999
1994
2006
2008
2009
2004
2000
1992
1995
1993
2002
2005
2003
2016
1997
1991
2014
2007
2001
2010
2012
2015
2013
2011
Figure 5: Annual incidence of dengue in Venezuela
(A) Annual dengue incidence (per 100 000 inhabitants) for the 1991–2016 period. Black vertical arrows indicate dengue epidemic years. Dotted black line indicates an
increasing linear trend (R²=0·27, t=2·99, p=0·006, n=26). Inset shows comparison of incidence data for Colombia, Brazil, and Venezuela. (B) Annual incidence by state
per 100 000 inhabitants from 1991 to 2016 (increasing from blue to red). *Central region. †Border region.
in incidence, frequency, and magnitude of dengue six increasingly large epidemics were recorded nationally
epidemics against a background of perennial endemic between 2007 and 2016, compared with four epidemics in
transmission. Dengue incidence has increased more than the previous 16 years.49 The largest epidemic occurred in
five-fold. The average incidence between 2010 and 2016 2010, when approximately 125 000 cases, including
was 211 cases per 100 000 population (figure 5A). Within 10 300 (8·24%) with severe manifestations, were
the country, the temporal increase in cases of dengue registered. During that year, Venezuela ranked third in the
mirrors the national dengue incidence, with regions of number of reported dengue cases in the American
higher population density (central regions) and those continent and second in the number of severe cases.50
bordering Colombia and Brazil (border regions) exhibiting The combination of poverty-related socioeconomic
a higher incidence (figure 5B). Worryingly, a total of factors, such as increasingly crowded living conditions,
8 www.thelancet.com/infection Published online February 21, 2019 http://dx.doi.org/10.1016/S1473-3099(18)30757-6
Review
growing population density, precarious homes, and long- syndrome incidence compared with the pre-Zika virus
lasting deficits in public services including frequent and baseline incidence—one of the highest (if not the
prolonged interruptions in water supply and electricity, highest) reported in the Americas.63 The number of
have been linked with a greater risk of acquiring dengue Guillain-Barré syndrome cases surged from a mean of
virus infection in Venezuela.51–54 These inadequacies have 214 annual cases reported before Zika virus, to more than
obliged residents to store water within households, 700 confirmed cases since the epidemic started.62 Cases
maintaining suitable breeding conditions for Ae aegypti of microcephaly and other congenital disorders related to
vectors during the dry season and throughout the year, Zika virus infections in pregnancy in Venezuela have
driving perennial dengue transmission. Additionally, the been reported, but the incidence remains to be
inability of vector control programmes to do proper determined by ongoing studies.
surveillance and timely application of vector control Beyond chikungunya, Zika, and dengue viruses, other
measures has caused the proportion of houses infested circulating arboviruses with epidemic potential exist in
with Ae aegypti to surpass the WHO transmission Venezuela. Outbreaks of Mayaro virus (often confused
threshold.55 Such conditions set the stage for subsequent with chikungunya) have been reported during the past
arboviral epidemics. decade.64,65 In 2010, Oropouche virus (of which there was
Venezuela was not spared from the havoc wrought by an outbreak in Peru in 2016) was detected in the
the epidemic of chikungunya in 2014, or the epidemic of Amazonian basin in South America, outside its typical
Zika virus a year later. The effect of these epidemics was distribution zone.66 The occurrence of epizootic strains of
amplified by the absence of timely official information, Venezuelan equine encephalitis virus and Madariaga
absence of preparedness, and the worsening economic virus (South American eastern equine encephalitis),67
and health crisis that resulted in acute shortages of along with cryptic transmission cycles of these viruses,
diagnostics medicines and medical supplies, and an when immunisation programmes have been halted, pose
overburdened health system. Both epidemics rapidly a further threat. No facilities exist for rapid laboratory
spread through densely inhabited regions where dengue diagnosis of either virus in Venezuela.68 The most
virus transmission was high. Although nationally, the common and effective Venezuelan equine encephalitis
attack rate of chikungunya was estimated to be between vaccine, TC-83, can no longer be bought or produced in
6·9% and 13·8%,56 the observed attack rate in populated Venezuela. The Agricultural Research Institute, with
urban areas reached 40–50%, similar to or higher than restricted production capacity, has no financial support
that reported in other countries.57,58 The total number of and production has been halted. Although there are no
chikungunya cases in Venezuela reported to PAHO in reliable official records of equine inventories, the threat of
2014 (by epidemiological week 52) was 30 405, with an latent outbreaks and their potential international dispersal
incidence of 112 per 100 000 population.59 Given the increases with the presence of wild donkeys without
paucity of official information since October, 2014, owners or sanitary control and persistent circulation of
estimates based on excess fever cases not explained epizootic Venezuelan equine encephalitis strains in
by another cause suggest that cases of chikungunya interepizootic periods in different sites of the plains and
exceeded 2 million, resulting in an incidence of the Catatumbo region, Venezuela.69
6975 cases per 100 000 population, more than 12 times Disease outbreaks associated with arbovirus are
the rate reported officially by the Venezuelan Ministry of increasing in frequency and magnitude throughout Latin
Health.56 Moreover, an important, yet unknown, number America. Although no evidence is available to suggest
of atypical and severe or fatal cases of chikungunya that the prevalence of certain arboviruses like dengue
occurred60 but were not reported by health personnel virus is higher in Venezuela than in other countries
because of fear of governmental reprisal.1,61 (figure 5A), the deficiency of public health infrastructure
In January, 2016, the Zika virus epidemic struck available for diagnosis and treatment is now a
Venezuela at the same time as a rise in dengue virus disproportionate problem in Venezuela compared with
transmission. The Zika virus outbreak evolved in a other countries in the region. Furthermore, given the
similar manner as chikungunya, rapidly affecting a high current situation, widespread under-reporting of cases by
proportion of the population. Inadequate preparedness comparison with other countries in the region is also
and deficiency of official communication once again was possible. Given previous patterns and the increase in risk
a cause for concern. The incidence of symptomatic cases factors favouring arboviral transmission, the abscence of
during the peak of the epidemic (the first 8 weeks of an increase in dengue incidence from 2014 onwards
2016) was estimated at 2124 cases per 100 000 population.62 suggests under-reporting.
Current estimates of serological (IgG) Zika virus Considering the precarious possibilities for cure of
positivity in pregnant women have reached roughly 80% arboviral diseases in Venezuela combined with the high
(Tami A, unpublished). As in other countries, an increase level of population displacement, emigrating infected
in the number of cases of Guillain-Barré syndrome was individuals could be unwittingly causing a spillover of
observed during the epidemic. However, Venezuela had these diseases to neighbouring countries, a process that
a rise of 877% (a 9·8-fold increase) in Guillain-Barré has not yet been quantified. The first major outbreak of
www.thelancet.com/infection Published online February 21, 2019 http://dx.doi.org/10.1016/S1473-3099(18)30757-6 9
Review
improvement and water management. This integrative
Search strategy and selection criteria approach differs little from current so-called best-practice
For malaria, Venezuelan and Latin American data were sourced prevention, control, and elimination of malaria. The
For more on PAHO see from the Pan American Health Organization (PAHO) Malaria success of the Venezuelan public health intervention
http://ais.paho.org Surveillance Indicators and Observatorio Venezolano de la helped to stimulate interest in global malaria elimination
For more on Observatorio Salud Documentos Oficiales. Brazilian border state data were during the 1960s.71
Venezolano de la Salud see Paradoxically, the onchocerciasis (a vector-borne hel
https://www.ovsalud.org
accessed from the Brazilian Ministry of Health Sistema de
Vigilância on May 5, 2018. Data for Colombian cases were minth infection) elimination programme in Venezuela
For more on the Sistema de
accessed via the Instituto Nacional de Salud Estadísticas has continued to work reasonably well. The programme’s
Vigilância see
http://200.214.130.44/sivep_ SIVIGILA 2017. Data on oral cases of Chagas disease in success is underpinned by the commitment and resolve
malaria/ Venezuela were sourced via PubMed from literature written in of its Venezuelan local health workers and indigenous
For more on the Instituto English or Spanish published between Jan 1, 2007 and health agents, and the regional support of the
Nacional de Salud see
May 25, 2018, and patient records at the Institute de Medicine Onchocerciasis Elimination Program for the Americas.2
http://www.ins.gov.co
Tropical, Caracas, Venezuela. Historical serological data for As a result of long-term mass drug administration with
Chagas disease in Venezuela and elsewhere were sourced via ivermectin on a biannual (and four times per year) basis
PubMed from literature published between Jan 1, 1950 and starting in 2000, interruption of onchocerciasis has been
May 25, 2018. Serological data for 2008–16 were derived from achieved among the northern foci located in the coastal
unpublished records at the Instituto de Medicina Tropical, mountain area,72 and parasite transmission now remains
Universidad Central de Venezuela, Caracas, Venezuela, and the in just 25% of the Venezuelan Yanomami of the southern
Centro de Medicina Tropical de Oriente, Universidad de Oriente Amazonian region.73 This regional initiative has proven
Núcleo Anzoátegui, Barcelona, Venezuela. Data for vector that the consensus of ministries of health, the endemic
abundance and infection rates (2014–16) were also derived communities, non-governmental organisations, and
from unpublished records at the Instituto de Medicina Tropical, public–private stakeholders, including WHO, is required
Universidad Central de Venezuela. Data for Colombian cases to develop and implement effective public health
were accessed from the Instituto Nacional de Salud Estadísticas programmes.2,71
SIVIGILA 2017. Human cutaneous leishmaniasis data from Venezuelans have endured a decade of political, social,
1990 to 2016 were sourced from the National Sanitary and economic upheaval that has left the country in crisis.
Dermatology programme of the Ministry of Health, available In addition to the return of measles and other vaccine-
from the Venezuelan Health Observatory (Observatorio preventable infectious diseases,74 conditions are favouring
Venezolano de la Salud). Data for Colombian cases were the unprecedented emergence and transmission of vector-
accessed from the Instituto Nacional de Salud Estadísticas borne diseases. Underpinning the current epidemics are
(El Sistema de Salud Pública) 2017. For dengue virus, inadequate surveillance, education, and awareness, and a
chikungunya, and Zika virus, we used the number of cases reduced capacity for effective inter vention. Successful
reported and notified by the Surveillance System of the control of the emerging crisis requires regional
Venezuelan Ministry of Health and Observatorio Venezolano coordination and, as we show in this Review, cross-border
de la Salud, at national level, the latter especially during the spillover is already ongoing, and expected to increase.
epidemics of chikungunya in 2014 and of Zika virus in Fortunately, many solutions are possible, even with
2015–2016. Latin American data were sourced from PAHO restricted resources. A good example is the successful
Dengue Surveillance Indicators. binational strategy for the elimination of malaria on the
Peru–Ecuador border. Collaboration at the operational
level included strengthening surveillance and training
dengue on the island of Madeira, an autonomous region community personnel to collect blood smears from
of Portugal, in 2012–13 is an example of the disease febrile people within their border communities,
export potential for Venezuela, as this outbreak was which prompted effective diagnosis, case definition
directly linked with a DENV-1 serotype from Venezuela.70 (indigenous, imported, introduced, induced, and cryptic),
and treatment.75 When state infrastructure does not work,
A call for action however, surveillance can be achieved via the mobilisation
For many decades, Venezuela was a leader in vector of citizen scientists and informal networks of health-care
control and public health policy in Latin America, even professionals. Technological advances in low-cost sample
more so after becoming the first WHO-certified country preservation, passive sampling, and in-situ diagnostics
to eliminate malaria in most of its territory in 1961.71 can also contribute. Education to raise awareness among
The interruption of malaria transmission was achieved communities at risk from disease can be achieved via
through systematic and integrative infection and social media, initiatives at schools, and information
vector control, case management, preventive diagnosis, campaigns at public centres. Surveillance data are
patient treatment, mass drug administration, community powerful and must be used as an advocacy tool to raise
participation through volunteer community health awareness among Venezuelan and regional authorities,
workers, and sanitary engineering such as housing and ultimately to encourage them to improve their
10 www.thelancet.com/infection Published online February 21, 2019 http://dx.doi.org/10.1016/S1473-3099(18)30757-6
Review
recognition of the growing crisis, cooperate, and accept 6 Tuite AR, Thomas-Bachli A, Acosta H, et al. Infectious disease
international medical interventions. Relevant inter implications of large-scale migration of Venezuelan nationals.
J Travel Med 2018; 25: 1–8.
national health authorities, such as the WHO Global 7 Nebehay S. Malaria on rise in crisis-hit Venezuela, WHO says.
Outbreak Alert and Response Network, must also take Reuters. 2018. https://www.reuters.com/article/us-health-malaria-
action to maintain accurate disease surveillance and venezuela/malaria-on-rise-in-crisis-hit-venezuela-who-says-
idUSKBN1HV1ON (accessed May 5, 2018).
response systems in the region along with collaboration 8 Avilán Rovira JM. El Boletín Epidemiológico Semanal.
with other strategic partners to provide timely Gac Méd Caracas 2008; 116: 1.
humanitarian assistance throughout this ongoing crisis. 9 El Nacional. Dejaron al país y a la OMS sin mapa de salud
The wider scientific community must support this venezolana. 2018. http://www.el-nacional.com/noticias/sociedad/
dejaron-pais-oms-sin-mapa-salud-venezolana_247285 (accessed
process by engaging with their Venezuelan and regional Sept 21, 2018).
colleagues, contributing to a robust, non-partisan 10 Pan American Health Organization. Epidemiological update—
evidence base for such interventions. Ultimately, national measles. WHO. 2018. https://www.paho.org/hq/index.
php?option=com_docman&task=doc_view&Itemid=270&gid=44328
and international political commitments are essential to &lang=en (accessed May 5, 2018).
stop a health crisis that threatens the whole region. 11 Recht J, Siqueira AM, Monteiro WM, et al. Malaria in Brazil,
The emergence, or re-emergence, of vector-borne Colombia, Peru and Venezuela: current challenges in malaria
control and elimination. Malar J 2017; 16: 273.
neglected diseases must be recognised as now extending 12 WHO. World malaria report 2018. 2018. https://www.who.int/
beyond the borders of Venezuela. These diseases have malaria/publications/world-malaria-report-2018/report/en/
already extended into neighbouring Brazil and Colombia, (accessed Feb 3, 2019).
13 Grillet ME, El Souki M, Laguna F, León JR. The periodicity of
and with increasing air travel and human migration, Plasmodium vivax and Plasmodium falciparum in Venezuela.
most of the Latin American and Caribbean region (as Acta Trop 2014; 129: 52–60.
well as some US cities hosting the Venezuelan diaspora, 14 Moreno Jorge E, Rubio-Palis Y, Martínez Ángela R, Porfirio A.
including Miami, FL, and Houston, TX) is at heightened Evolución espacial y temporal de la malaria en el municipio
Sifontes del estado Bolívar, Venezuela. 1980–2013.
risk for disease re-emergence. Accordingly, we call on the Bol Mal Salud Amb 2014; 54: 236–49.
members of the Organization of American States and 15 Foster PG, de Oliveira TMP, Bergo ES, et al. Phylogeny of
other international political bodies to become better and Anophelinae using mitochondrial protein coding genes.
R Soc Open Sci 2017; 4: 170758.
more effectively engaged in strengthening Venezuela’s 16 Moreno JE, Rubio-Palis Y, Páez E, Pérez E, Sánchez V. Abundance,
now-depleted health system by applying more pressure biting behaviour and parous rate of anopheline mosquito species in
to the government to accept humanitarian assistance relation to malaria incidence in gold-mining areas of southern
Venezuela. Med Vet Entomol 2007; 21: 339–49.
offered by the international community.2 Without such 17 Abou Orm S, Moreno JE, Carrozza M, Acevedo P, Herrera F.
international interventions, the possibility that the public Plasmodium spp. infection rates for some Anopheles spp. from
health gains achieved over the past 18 years, through Sifontes Municipality, Bolívar State, Venezuela. Bol Mal Salud Amb
2017; 57: 17–25.
Millennium Development Goal 6 (to combat AIDS,
18 Moreno JE, Rubio-Palis Y, Sánchez V, Martínez Á. Caracterización
malaria, and other diseases) and the new Sustainable de hábitats larvales de anofelinos en el municipio Sifontes del
Development Goals, could be soon reversed is real. estado Bolívar, Venezuela. Bol Mal Salud Amb 2015; 55: 117–31.
19 Grillet ME, Villegas L, Oletta JF, Tami A, Conn JE. Malaria in
Contributors Venezuela requires response. Science 2018; 359: 528.
All authors were involved in the writing of the Review or data analysis
20 Wide A, Pabón R, De Abreu N, et al. Prevalence of asymptomatic
and figure preparations, or both. Plasmodium vivax infections in the North-Eastern focus of malaria
Declaration of interests of Venezuela. Bol Mal Salud Amb 2016; 56: 160–71 (in Spanish).
We declare no competing interests. 21 Pérez-Molina JA, Molina I. Chagas disease. Lancet 2018; 391: 82–94.
22 Yeo M, Acosta N, Llewellyn M, et al. Origins of Chagas disease:
Acknowledgments didelphis species are natural hosts of Trypanosoma cruzi I and
This work was supported in part by the Scottish Funding Council Global armadillos hosts of Trypanosoma cruzi II, including hybrids.
Challenges Research Fund, (Small Grants Fund SFC/AN/12/2017). Int J Parasitol 2005; 35: 225–33.
The authors would like to dedicate this work to the memory of Xoan 23 Añez N, Crisante G, Rojas A. Update on Chagas disease in
Noya Alarcon. Venezuela—a review. Mem Inst Oswaldo Cruz 2004; 99: 781–87.
References 24 Acquatella H. Estado actual de la enfermedad de Chagas en
1 Burki T. Re-emergence of neglected tropical diseases in Venezuela. Venezuela y de su manejo terapéutico. Gac Méd Caracas 2003;
Lancet Infect Dis 2015; 15: 641–42. 111: 136–56.
2 Hotez PJ, Basáñez MG, Acosta-Serrano A, Grillet ME. Venezuela 25 Aché A, Matos AJ. Interrupting Chagas disease transmission in
and its rising vector-borne neglected diseases. PLoS Negl Trop Dis Venezuela. Rev Inst Med Trop Sao Paulo 2001; 43: 37–43.
2017; 11: e0005423. 26 Rodríguez-Bonfante C, Amaro A, García M, et al. Epidemiology of
3 Du R, Stanaway J, Hotez PJ. Could violent conflict derail the London Chagas disease in Andrés Eloy Blanco, Lara, Venezuela: triatomine
declaration on NTDs? PLoS Negl Trop Dis 2018; 12: e0006136. infestation and human seroprevalence. Cad Saude Publica 2007;
23: 1133–40 (in Spanish).
4 United Nations Refugee Agency. As Venezuelans flee throughout
Latin America, UNHCR issues new protection guidance. 2018. 27 Bonfante-Cabarcas R, Rodríguez-Bonfante C, Vielma BO, et al.
http://www.unhcr.org/news/briefing/2018/3/5aa793c14/ Seroprevalence for Trypanosoma cruzi infection and associated
venezuelans-flee-throughout-latin-america-unhcr-issues-new- factors in an endemic area of Venezuela. Cad Saude Publica 2011;
protection-guidance.html (accessed May 5, 2018). 27: 1917–29.
5 United States Agency for International Development. Research and 28 Cardozo E. Mal de Chagas deja ocho muertos y 40 infectados en
development progress report. 2018. https://www.usaid.gov/ Táchira. La Opinion. 2018. https://www.laopinion.com.co/
GlobalDevLab/documents/research-and-development-progress- venezuela/mal-de-chagas-deja-ocho-muertos-y-40-infectados-en-
report/fy-2015 (accessed May 5, 2018). tachira-152365#OP (accessed May 5, 2018).
www.thelancet.com/infection Published online February 21, 2019 http://dx.doi.org/10.1016/S1473-3099(18)30757-6 11
Review
29 Cucunubá ZM, Nouvellet P, Conteh L, et al. Modelling historical 50 Pan American Health Organization. Reported cases of dengue fever
changes in the force-of-infection of Chagas disease to inform in the Americas by country or territory. WHO. 2018. http://www.
control and elimination programmes: application in Colombia. paho.org/data/index.php/en/mnu-topics/indicadores-dengue-en/
BMJ Glob Health 2017; 2: e000345. dengue-nacional-en/252-dengue-pais-ano-en.html (accessed
30 Black CL, Ocaña-Mayorga S, Riner DK, et al. Seroprevalence Jan 15, 2019).
of Trypanosoma cruzi in rural Ecuador and clustering of 51 Barrera R, Navarro JC, Mora JD, Domínguez D, González J.
seropositivity within households. Am J Trop Med Hyg 2009; Public service deficiencies and Aedes aegypti breeding sites in
81: 1035–40. Venezuela. Bull Pan Am Health Organ 1995; 29: 193–205.
31 Noya BA, Díaz-Bello Z, Colmenares C, et al. Update on oral Chagas 52 Barrera R, Delgado N, Jiménez M, Villalobos I, Romero I.
disease outbreaks in Venezuela: epidemiological, clinical and Stratification of a hyperendemic city in hemorrhagic dengue.
diagnostic approaches. Mem Inst Oswaldo Cruz 2015; 110: 377–86. Rev Panam Salud Publica 2000; 8: 225–33.
32 Alarcón de Noya B, Ruiz-Guevara R, Diaz-Bello Z, et al. 53 Velasco-Salas ZI, Sierra GM, Guzmán DM, et al.
En Venezuela la enfermedad de Chagas de transmisión oral llegó Dengue seroprevalence and risk factors for past and recent viral
para quedarse. 2016. https://www.tribunadelinvestigador.com/ transmission in Venezuela: a comprehensive community-based
ediciones/2016/2/art-7/ (accessed May 25, 2018). study. Am J Trop Med Hyg 2014; 91: 1039–48.
33 Noya O, Ruiz-Guevara R, Diaz-Bello Z, Alarcón de Noya B. 54 Vincenti-Gonzalez MF, Grillet ME, Velasco-Salas ZI, et al.
Epidemiología y clínica de la transmisión oral de Trypanosoma cruzi. Spatial analysis of dengue seroprevalence and modeling of
2015. https://issuu.com/isglobal/docs/revista_espa__ola_de_salud_ transmission risk factors in a dengue hyperendemic city of
publica_ (accessed May 25, 2018). Venezuela. PLoS Negl Trop Dis 2017; 11: e0005317.
34 Alarcón de Noya B, Díaz-Bello Z, Colmenares C, et al. Eficacia 55 Grillet ME, Del Ventura. Emergencia del Zika en Latinoamerica y
terapéutica en el primer brote de transmisión oral de la enfermedad Control de Aedes aegypti. Bol Dir Malariol Saneam Ambient 2016;
de Chagas en Venezuela. Biomédica 2011; 31: 64–65. 56: 97–102.
35 Carrasco HJ, Torrellas A, García C, Segovia M, Feliciangeli MD. 56 Oletta JF. Epidemia de fiebre chikungunya en Venezuela, 2014–2015.
Risk of Trypanosoma cruzi I (Kinetoplastida: Trypanosomatidae) Gac Méd Caracas 2016; 124: 122–37.
transmission by Panstrongylus geniculatus (Hemiptera: Reduviidae) 57 Schwartz O, Albert ML. Biology and pathogenesis of chikungunya
in Caracas (Metropolitan District) and neighboring States, virus. Nat Rev Microbiol 2010; 8: 491–500.
Venezuela. Int J Parasitol 2005; 35: 1379–84. 58 Pimentel R, Skewes-Ramm R, Moya J. Chikungunya in the
36 Carrasco HJ, Segovia M, Londoño JC, Ortegoza J, Rodríguez M, Dominican Republic: lessons learned in the first six months.
Martínez CE. Panstrongylus geniculatus and four other species of Rev Panam Salud Publica 2014; 36: 336–41.
triatomine bug involved in the Trypanosoma cruzi enzootic cycle: 59 Pan American Health Organization. Number of reported cases of
high risk factors for Chagas’ disease transmission in the chikungunya fever in the Americas, by country or territory
Metropolitan District of Caracas, Venezuela. Parasit Vectors 2014; 2013–2014. Epidemiological week 52. WHO. Dec 29, 2014.
7: 602. https://www.paho.org/hq/dmdocuments/2014/2014-dec-29-cha-
37 Segovia M, Carrasco HJ, Martínez CE et al. Molecular CHIKV-cases-ew-52.pdf (accessed June 20, 2018).
epidemiologic source tracking of orally transmitted Chagas disease, 60 Torres JR, Leopoldo Códova G, et al. Chikungunya fever: atypical and
Venezuela. Emerg Infect Dis 2013; 19: 1098–101. lethal cases in the western hemisphere: a Venezuelan experience.
38 Incani RN. Leishmaniasis y sus vectores. In: Incani RN, ed. IDCases 2014; 2: 6–10.
Parasitology. Valencia, Venezuela: Universidad de Carabobo Press, 61 Wade L. Scientific community. For Venezuelan academics, speaking
2010: 161–76. out is risky business. Science 2014; 346: 287.
39 Guevara JR. Estadísticas de Leishmaniasis cútanea en Venezuela 62 Oletta JF. 2016. Evolución de la epidemia de Zika en Venezuela,
Historico / Año 1990–2016. Archives of the Ministerio de Poder hasta el 21 de marzo de 2016. Gac Méd Caracas 2016; 124: 50–56.
Popular Para la Salud, Venezuela, Servicio Autónomo Instituto de
63 Dos Santos T, Rodriguez A, Almiron M, et al. Zika virus and the
Biomedicina. Caracas, Venezuela: Ministerio de Poder Popular Para
Guillain-Barré syndrome—case series from seven countries.
la Salud, Venezuela official press, 2016.
N Engl J Med 2016; 375: 1598–601.
40 Lugo DA, Ortega-Moreno ME, Rodriguez V, et al. Seroprevalence of
64 Auguste AJ, Liria J, Forrester NL, et al. Evolutionary and ecological
canine visceral leishmaniasis by rK39-ELISA in endemic foci in
characterization of Mayaro virus strains isolated during an outbreak,
Venezuela. Rev Fac Cs Vets 2015; 56: 42–51.
Venezuela, 2010. Emerg Infect Dis 2015; 21: 1742–50.
41 Seaman J, Mercer AJ, Sondorp E. The epidemic of visceral
65 Muñoz M, Navarro JC. Mayaro: a re-emerging arbovirus in
leishmaniasis in western Upper Nile, southern Sudan: course and
Venezuela and Latin America. Biomedica 2012; 32: 286–302.
impact from 1984 to 1994. Int J Epidemiol 1996; 25: 862–71.
66 Navarro JC, Giambalvo D, Hernandez R, et al. Isolation of Madre de
42 Patino LH, Mendez C, Rodriguez O, et al. Spatial distribution,
Dios virus (Orthobunyavirus; Bunyaviridae), an Oropouche virus
leishmania species and clinical traits of cutaneous leishmaniasis
species reassortant, from a monkey in Venezuela.
cases in the Colombian army. PLoS Negl Trop Dis 2017; 11: e0005876.
Am J Trop Med Hyg 2016; 95: 328–38.
43 Sharara SL, Kanj SS. War and infectious diseases: challenges of the
67 Blohm GM, Lednicky JA, White SK, et al. Madariaga virus:
Syrian civil war. PLoS Pathog 2014; 10: e1004438.
identification of a lineage III strain in a Venezuelan child with acute
44 Instituto Nacional de Salud. Estadísticas SIVIGILA 2017. 2017. undifferentiated febrile illness, in the setting of a possible equine
https://www.ins.gov.co/Direcciones/Vigilancia/Lineamientosy epizootic. Clin Infect Dis 2018; 67: 619–21.
documentos/Lineamientos%202018.pdf (accessed May 5, 2018).
68 Rodriguez-Morales AJ, Culquichicón C. The need for enhancing the
45 Hotez PJ, Murray KO. Dengue, West Nile virus, chikungunya, message: screening for Zika, STORCH, and other agents and
Zika-and now Mayaro? PLoS Negl Trop Dis 2017; 11: e0005462. co-infections should be considered and assessed. Am J Reprod
46 Braack L, Gouveia de Almeida AP, Cornel AJ, Swanepoel R, Immunol 2017; 78: e12688.
de Jager C. Mosquito-borne arboviruses of African origin: review of 69 Navarro JC, Medina G, Vasquez C et al. Postepizootic persistence of
key viruses and vectors. Parasit Vectors 2018; 11: 29. Venezuelan equine encephalitis virus, Venezuela. Emerg Infect Dis
47 Ferguson NM, Cucunubá ZM, Dorigatti I, Nedjati-Gilani GL, 2005; 11: 1907–15.
Donnelly CA, Basáñez MG. Countering the Zika epidemic in Latin 70 Franco L, Pagan I, Serre Del Cor N, et al. Molecular epidemiology
America. Science 2016; 353: 353–54. suggests Venezuela as the origin of the dengue outbreak in Madeira,
48 WHO. Dengue guidelines for diagnosis, treatment, prevention and Portugal in 2012–2013. Clin Microbiol Infect 2015; 21: 713.e5–8.
control. World Health Organization. 2009. http://www.who.int/tdr/ 71 Griffing SM, Villegas L, Udhayakumar V. Malaria control and
publications/documents/dengue-diagnosis.pdf (accessed elimination, Venezuela, 1800s–1970s. Emerg Infect Dis 2014;
May 5, 2018). 20: 1697–704.
49 Vincenti-Gonzalez MF, Tami A, Lizarazo EF, Grillet ME. 72 Convit J, Schuler H, Borges R et al. Interruption of Onchocerca
ENSO-driven climate variability promotes periodic major volvulus transmission in northern Venezuela. Parasit Vectors 2013;
outbreaks of dengue in Venezuela. Sci Rep 2018; 8: 5727. 6: 289.
12 www.thelancet.com/infection Published online February 21, 2019 http://dx.doi.org/10.1016/S1473-3099(18)30757-6
Review
73 Botto C, Basañez MG, Escalona M et al. Evidence of suppression of 75 Krisher LK, Krisher J, Mariano Ambulidu, et al. Successful malaria
onchocerciasis transmission in the Venezuelan Amazonian focus. elimination in the Ecuador–Peru border region: epidemiology and
Parasit Vectors 2016 27; 9: 40. lessons learned. Malar J 2016; 15: 573.
74 Paniz-Mondolfi A, Tami A, Grillet ME, et al. Resurgence of vaccine-
preventable diseases in Venezuela as a regional public health threat © 2019 Elsevier Ltd. All rights reserved.
in the Americas. Emerg Infect Dis 2019; published online Jan 6.
DOI:10.3201/eid2504.181305 (preprint).
www.thelancet.com/infection Published online February 21, 2019 http://dx.doi.org/10.1016/S1473-3099(18)30757-6 13
View publication stats
You might also like
- Lumbar Disc Herniation - Franco Postacchini - Springer Verlag Wien (1999)Document628 pagesLumbar Disc Herniation - Franco Postacchini - Springer Verlag Wien (1999)VineethJosephNo ratings yet
- Hip Spica Nursing CareDocument10 pagesHip Spica Nursing CareItstineeNo ratings yet
- Assignment 2Document4 pagesAssignment 2JafinNo ratings yet
- Tip: Urinary Proteins Less Than 300 MG Is Not Detectable by Urine DipstickDocument11 pagesTip: Urinary Proteins Less Than 300 MG Is Not Detectable by Urine DipstickAsif Newaz100% (2)
- 1 s2.0 S0140673623017877 MainDocument16 pages1 s2.0 S0140673623017877 Mainybahos3misenaNo ratings yet
- Grillet 2018 Letter To Science Malaria in Venezuela Requaires ResponseDocument3 pagesGrillet 2018 Letter To Science Malaria in Venezuela Requaires ResponseR. AltezNo ratings yet
- Dzul-Manzanilla Et Al 2021Document9 pagesDzul-Manzanilla Et Al 2021Ana Beatriz Vasquez RodriguezNo ratings yet
- Dengue LancetDocument16 pagesDengue LancetJorge Ricardo Uchuya GómezNo ratings yet
- Colunga-Salas Et Al. 2020Document13 pagesColunga-Salas Et Al. 2020Ana RengifoNo ratings yet
- La Pandemia - Luysana Moran.Document5 pagesLa Pandemia - Luysana Moran.Luysana MoranNo ratings yet
- ¿Qué Dicen Los Números de La Evolución Temporal de La Enfermedad de Chagas?Document8 pages¿Qué Dicen Los Números de La Evolución Temporal de La Enfermedad de Chagas?rachell cockerNo ratings yet
- WJG 27 1691Document26 pagesWJG 27 1691Valentina QuinteroNo ratings yet
- Carvalho, 2018Document13 pagesCarvalho, 2018neneNo ratings yet
- Dengue (Lancet)Document16 pagesDengue (Lancet)Roberth Mero100% (1)
- Practiva No 3 Anova Serrano 2017Document7 pagesPractiva No 3 Anova Serrano 2017CARLOS DIONEY MOLINA CLEMENTENo ratings yet
- HIV in Iran: CorrespondenceDocument1 pageHIV in Iran: CorrespondenceSebastián BarragánNo ratings yet
- PLOSONE Journal - Pone.0241579 SYNDocument14 pagesPLOSONE Journal - Pone.0241579 SYNRus NaingNo ratings yet
- Are Leprosy Case Numbers Reliable?: CommentDocument3 pagesAre Leprosy Case Numbers Reliable?: CommentMedNo ratings yet
- Cidacs 100milhoesDocument11 pagesCidacs 100milhoesJessé NóbregaNo ratings yet
- Mapping The Burden of Cholera in Sub-Saharan Africa and Implications For Control: An Analysis of Data Across Geographical ScalesDocument8 pagesMapping The Burden of Cholera in Sub-Saharan Africa and Implications For Control: An Analysis of Data Across Geographical ScalesMasithaNo ratings yet
- Management and Education in Patients With Diabetes Mellitus: January 2017Document14 pagesManagement and Education in Patients With Diabetes Mellitus: January 2017SUSANTONo ratings yet
- Maternal, Fetal, and Neonatal Outcomes in Pregnant Dengue Patients in MexicoDocument8 pagesMaternal, Fetal, and Neonatal Outcomes in Pregnant Dengue Patients in MexicoLola del carmen RojasNo ratings yet
- Infection, Genetics and Evolution: Contents Lists Available atDocument10 pagesInfection, Genetics and Evolution: Contents Lists Available atDIEGO FERNANDO TULCAN SILVANo ratings yet
- Nejmoa 2107715Document10 pagesNejmoa 2107715carb0ne14rNo ratings yet
- 0104 1169 Rlae 27 E3160Document10 pages0104 1169 Rlae 27 E3160Rafaella AranhaNo ratings yet
- Journal LeticiaDocument12 pagesJournal LeticiaDewi KUNo ratings yet
- Baby Research (Edited 2)Document15 pagesBaby Research (Edited 2)liezel de la cernaNo ratings yet
- 2021 - Acute Chagas Disease in ColombiaDocument14 pages2021 - Acute Chagas Disease in ColombiaDaniel Velázquez-RamírezNo ratings yet
- COVID 19 PandemicDocument4 pagesCOVID 19 PandemiccatmewNo ratings yet
- ArtigosDocument7 pagesArtigosLeoberto TorresNo ratings yet
- Shearer 2018Document9 pagesShearer 2018Dreyara ElleNo ratings yet
- 0031Document7 pages0031Julian BesoniNo ratings yet
- Depression and Associated Factos in Medical Students in Acapulco During The COVID-19 PandemicDocument15 pagesDepression and Associated Factos in Medical Students in Acapulco During The COVID-19 PandemicEsteban MatusNo ratings yet
- El NiñoDocument2 pagesEl Niñojdpq47qx8tNo ratings yet
- Rosser Et-Al 2022Document16 pagesRosser Et-Al 2022aaraujodefNo ratings yet
- 1 s2.0 S003335061630436X MainDocument7 pages1 s2.0 S003335061630436X MainMardaniah HasanNo ratings yet
- Heliyon: A.D. Domínguez-Gonz Alez, G. Guzm An-Valdivia, F.S. Angeles-T Ellez, M.A. Manjarrez-Angeles, R. Secín-DiepDocument5 pagesHeliyon: A.D. Domínguez-Gonz Alez, G. Guzm An-Valdivia, F.S. Angeles-T Ellez, M.A. Manjarrez-Angeles, R. Secín-DiepAlejandro MartinezNo ratings yet
- Lancet Venezuela Health CrisisDocument1 pageLancet Venezuela Health CrisisLuis Carlos OlivarNo ratings yet
- Dengue and Dengue Hemorrhagic Fever Among Adults CDocument9 pagesDengue and Dengue Hemorrhagic Fever Among Adults CSadio KeitaNo ratings yet
- ScedosporiumDocument12 pagesScedosporiumaiturraldeNo ratings yet
- Chagas Disease: SeminarDocument13 pagesChagas Disease: SeminarRafaelNo ratings yet
- 25epidemimex2 PDFDocument20 pages25epidemimex2 PDFIDEAS MEDICINA by ArthurNo ratings yet
- Gender Differences, Inequalities and Biases in The Management of Acute Coronary SyndromeDocument14 pagesGender Differences, Inequalities and Biases in The Management of Acute Coronary SyndromeDaniela BlancoNo ratings yet
- Lexicometric and Sentiment Analysis of News in The Spanish Press Regarding Hiv and PrisonDocument6 pagesLexicometric and Sentiment Analysis of News in The Spanish Press Regarding Hiv and PrisonHerald Scholarly Open AccessNo ratings yet
- Latinamericanist: Health and Policy in Latin America and The CaribbeanDocument20 pagesLatinamericanist: Health and Policy in Latin America and The CaribbeanEllie SintjagoNo ratings yet
- Diagnostics For COVID-19Document12 pagesDiagnostics For COVID-19JOHN WALSHNo ratings yet
- Articles: BackgroundDocument9 pagesArticles: BackgroundjclamasNo ratings yet
- Rift Valley Fever (RVF) : Is There A Global Concern On This Emerging Zoonotic and Vector-Borne Disease?Document2 pagesRift Valley Fever (RVF) : Is There A Global Concern On This Emerging Zoonotic and Vector-Borne Disease?FlabberNo ratings yet
- Lancet BFseriespaper1Document17 pagesLancet BFseriespaper1nosh.rose95No ratings yet
- A Literature Review On Current Tropical Diseases and The Role of Pharmacist in Public Health With Special Reference To Tropical DiseasesDocument8 pagesA Literature Review On Current Tropical Diseases and The Role of Pharmacist in Public Health With Special Reference To Tropical DiseasesmehakNo ratings yet
- Challenges in Dengue Vaccines Development: Pre-Existing Infections and Cross-ReactivityDocument15 pagesChallenges in Dengue Vaccines Development: Pre-Existing Infections and Cross-ReactivityNandagopal PaneerselvamNo ratings yet
- Aetiology and Frequency of Cervico-Vaginal Infections Among Mexican WomenDocument8 pagesAetiology and Frequency of Cervico-Vaginal Infections Among Mexican WomenMiriam SuarezNo ratings yet
- Fmicb 11 631736Document16 pagesFmicb 11 631736Herick Lyncon Antunes RodriguesNo ratings yet
- Review Article: Chagas' Disease: Pregnancy and Congenital TransmissionDocument11 pagesReview Article: Chagas' Disease: Pregnancy and Congenital Transmissiondiaa skamNo ratings yet
- What To Expect From The 2017 Yellow Fever Outbreak in Brazil?Document4 pagesWhat To Expect From The 2017 Yellow Fever Outbreak in Brazil?Johnny Saavedra CamachoNo ratings yet
- 1-s2.0-S2529993X2100201X-main EMBASEDocument13 pages1-s2.0-S2529993X2100201X-main EMBASEybahos3misenaNo ratings yet
- PEÑA-RODRIGUEZ2023 Prevalence of Symptoms, Comorbidities, and Reinfections in Individuals Infected With Wild-Type SARS-CoV-2Document9 pagesPEÑA-RODRIGUEZ2023 Prevalence of Symptoms, Comorbidities, and Reinfections in Individuals Infected With Wild-Type SARS-CoV-2Oliver Viera SeguraNo ratings yet
- Market Integration and Soil-Transmitted Helminth Infection Among The Shuar of Amazonian EcuadorDocument24 pagesMarket Integration and Soil-Transmitted Helminth Infection Among The Shuar of Amazonian EcuadorDaffa Ahmad NaufalNo ratings yet
- Nature 2020 03 05Document467 pagesNature 2020 03 05Desmond pengNo ratings yet
- PIIS0140673619325243Document2 pagesPIIS0140673619325243blue diamondNo ratings yet
- Detection of Novel Coronaviruses in Bats in Myanmar: A1111111111 A1111111111 A1111111111 A1111111111 A1111111111Document11 pagesDetection of Novel Coronaviruses in Bats in Myanmar: A1111111111 A1111111111 A1111111111 A1111111111 A1111111111VoskiNo ratings yet
- Accepted Manuscript: Acta TropicaDocument49 pagesAccepted Manuscript: Acta TropicaIvonne CabreraNo ratings yet
- 66 EngDocument10 pages66 EngAnonymous JUPZazcr1nNo ratings yet
- The Bartonellas and Peruvian Medicine: The Work of Alberto Leonardo BartonFrom EverandThe Bartonellas and Peruvian Medicine: The Work of Alberto Leonardo BartonNo ratings yet
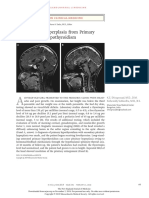
- Pituitary Hyperplasia From Primary Hypothyroidism: Images in Clinical MedicineDocument1 pagePituitary Hyperplasia From Primary Hypothyroidism: Images in Clinical MedicinedanielNo ratings yet
- Pti PurpuraDocument10 pagesPti PurpuradanielNo ratings yet
- Switch 2Document12 pagesSwitch 2danielNo ratings yet
- New Guidelines For Statistical Reporting in The: EditorialsDocument2 pagesNew Guidelines For Statistical Reporting in The: EditorialsdanielNo ratings yet
- Comment: Vs Failure of Implementation), Which Can Provide UniqueDocument2 pagesComment: Vs Failure of Implementation), Which Can Provide UniquedanielNo ratings yet
- Surgical Patient Care - Improving Safety, Quality and ValueDocument926 pagesSurgical Patient Care - Improving Safety, Quality and ValueYen Ngo100% (2)
- Diabetic KetoacidosisDocument38 pagesDiabetic KetoacidosisEdelrose LapitanNo ratings yet
- Transforming Medical EducationDocument3 pagesTransforming Medical EducationMob Man3No ratings yet
- 13029-Article Text-47609-1-10-20121220 PDFDocument4 pages13029-Article Text-47609-1-10-20121220 PDFtanmai nooluNo ratings yet
- Tumour Lysis SyndromeDocument15 pagesTumour Lysis SyndromeziggyshahdustNo ratings yet
- The Importance of Oral Health in Palliative Care Patients: Emma RileyDocument5 pagesThe Importance of Oral Health in Palliative Care Patients: Emma RileyShinta Dewi NNo ratings yet
- A LZHEIMER'SDocument9 pagesA LZHEIMER'SPaul Mark PilarNo ratings yet
- Study Data Reviewer's Guide: LDCP, Inc. Study LDCP-0242-005Document16 pagesStudy Data Reviewer's Guide: LDCP, Inc. Study LDCP-0242-005anon_181306460No ratings yet
- Developing Mental Health-Care Quality Indicators: Toward A Common FrameworkDocument6 pagesDeveloping Mental Health-Care Quality Indicators: Toward A Common FrameworkCarl FisherNo ratings yet
- Drug Card Emergency Department-1 PDFDocument2 pagesDrug Card Emergency Department-1 PDFdrmohdtanveerNo ratings yet
- Dysphagia: Dr. Sameeah A. Rashid MBCHB, DMRD, Fibms College of Medicine, Hawler Medical UniversityDocument35 pagesDysphagia: Dr. Sameeah A. Rashid MBCHB, DMRD, Fibms College of Medicine, Hawler Medical UniversityDarawan MirzaNo ratings yet
- Asthma - Peak Flow MeterDocument22 pagesAsthma - Peak Flow Meterapi-3729824100% (2)
- Pre-Op Preparation and Assessment of Pediatric PatientsDocument62 pagesPre-Op Preparation and Assessment of Pediatric PatientsBedahanakugmNo ratings yet
- Waiters Newborn Care PDFDocument1 pageWaiters Newborn Care PDFmp1757No ratings yet
- Vision 2020 CollectionDocument272 pagesVision 2020 CollectionFandyNo ratings yet
- HomeYour LibraryDocument47 pagesHomeYour LibraryMary Margareth GonzalesNo ratings yet
- Edukasi Keluarga Tentang OralitDocument8 pagesEdukasi Keluarga Tentang OralitRaudhiatul AzzahraNo ratings yet
- 07 RJR 08 Particularities of Anesthesia in ENT Endoscopic SurgeryDocument8 pages07 RJR 08 Particularities of Anesthesia in ENT Endoscopic SurgeryAyuAnatrieraNo ratings yet
- Cough Management: A Practical ApproachDocument13 pagesCough Management: A Practical ApproachJonathan OverianNo ratings yet
- Laporan Pemakaian Dan Lembar Permintaan Obat Puskesmas Kab. Tapanuli TengahDocument10 pagesLaporan Pemakaian Dan Lembar Permintaan Obat Puskesmas Kab. Tapanuli TengahVhaa OliviaNo ratings yet
- 866-Article Text-6088-1-10-20210107Document8 pages866-Article Text-6088-1-10-20210107GusrianiNo ratings yet
- Notes On Research ProblemDocument9 pagesNotes On Research ProblemRadhaNo ratings yet
- Local-Investment-Plan-2023-2025 SNDocument12 pagesLocal-Investment-Plan-2023-2025 SNFerdinand M. DancelandNo ratings yet
- Quality of Life Impairment in Depression and Anxiety DisordersDocument8 pagesQuality of Life Impairment in Depression and Anxiety DisordersRiham AmmarNo ratings yet
- Guidelines For Valve QuantificationDocument1 pageGuidelines For Valve QuantificationZoltán Tirczka100% (1)
- Prelim MtlbeDocument6 pagesPrelim MtlbeRachel Ann Eromon100% (1)