Professional Documents
Culture Documents
Epoxyethane (Ethylene Oxide)
Uploaded by
li xian0 ratings0% found this document useful (0 votes)
11 views3 pagesEpoxyethane
Copyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentEpoxyethane
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
11 views3 pagesEpoxyethane (Ethylene Oxide)
Uploaded by
li xianEpoxyethane
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You are on page 1of 3
The Essential Chemical Industry - online
Cig
search
te ei
®
Epoxyethane (Ethylene oxide)
Epoxyethane (ethylene oxide, EO) is a colourless gas at room temperature. The bonds inthe ring are easily broken so °.
epoxyethane is very reactive. Ths, together with it being made directly from ethene (ethylene), a readily available
feedstock, makes it an extremely important intermediate in the manufacture of many useful chemicals,
\
Hc’—cH,
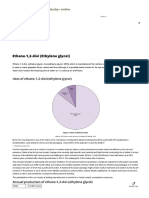
Uses of epoxyethane (ethylene oxide)
‘The principal use of epoxyetrane isn the procucton of ethane-t,2-do, the principal consttuent of engine coolants, and in other dos the
{1/CO}), which are used to make polyesters. These and other chemicals made ftom epoxyethane are dlscussed below.
BZ eovervone anes
2
‘Figure 4 Uses ofepoxythane
a om may sore mn lb Prati, 216; Marketa Raa Conn. 206
Figur 1 shows th wore aes of epoxyetane By proportion. Butheve vary county by coun. For xampl ch a, by operon is
Used nthe US to make the dl (41%) and mor is sed to make gyal ehars(10%).ethoxyaes (15%), thanclamines (18%) and povetiyene
aoa (12%
Annual production of epoxyethane (ethylene oxide)
[wen [2+ miton tonnes?
[asia_| 0.4 milion tonnes?
Us, | 25 nition tonne
Dao
Matern ac Contin 218, Rage fr 202
2: psd rach 6 nr by 3018
Manufacture of epoxyethane (ethylene oxide)
Ethene is mixed with airor oxygen and passed over a catalyst ely divided siver on an inert support such as alumina) at 620-550 K and under
16.20 atmospheres pressure (Figure 2). Two reactions, paral and complete oxidation, take place s:nukaneously at the catalyst surface, A small
‘amount of 12-dehloroethane is added tothe reaction mixture which decreases the unwanted side reaction to carbon dioxide and steam.
Residence tein he reactors 1-4 seconds.
‘SWveris unique as a catalyst‘or this reacton but he mechanism isnot clear. The selectivity now being achieved is over 90%. As the catalyst
ages, seleciy decreases. The Hetme of catalyst isin the range of -5 years
1402
9,
H.C —'cH,
hcxon, B® = 1007 kd me
‘rene
32. 200, +2H,0
BH? = 1300 4 met!
‘Figure 2 Two competing reactions forthe oxidation of ethane.
(The entlpy values ar 523K and 45 atm)
Reactions of epoxyethane (ethylene oxide)
Production and uses of ethylene glycols
Epoxyetnane is eactd wih walerunder neal or ace ondtons, to frm stepwise a range of products:
°
nH;e —ch, + H,0
e
n=? HO—CH,CH,0—H
(wanseror ope)
HO-CH,CH,O-CH,CH,0—H
etme ao!
HO—CH,CH,0—CH,CH,0—CH,CH,0—H
nampene ae
HO-(CH,CH,0),-H
ayron gyeoPEG)
Figure 3 Production of ethylene pico.
When n= 1, the productis ethane-1,2-ol chemicalsethane-12diol.him) often known as monoethylene glycol) HOCH;CH,OH, used in engine
coolants in heat ransfer fis, and for production of polyesters such as PET {/palymersipolyesters. him) (polyethylene terephthalate),
When n= 2, the product is HOCH,CH,OCH,CH,OH, usualy krown as diatylane glycol tis used principally ta mab
(ipolymersipolyurethane.him) and polyesters (pelymersipalyestersnim).
Whon n= 3, the products used as a plaster (polymersipolymers-an-overviow.himbtilor
polyurethanes
Figure 4 Ebene.
Uehemicateetrane 2am)
and detyene glyco, Both
‘made rom epoxyetrane 2s wll
«8 propanedl, are used ode
Jee arr prior to takeoff in
old conatons. I this phot,
(he wings of an Air Cansce
‘Alrbus A320 are bong dolce.
hd emus of eon
(Pm Common
When n =4 or more, the productis a poly(epoxyethane), known usually as polyethylene glycol (PEG). These are classified by relative molecular
‘mass, PEG200, PEG400, PEGEOO, alc and are used as nonionic surfactants (materals-and-applcationsisufacians.himlénonionic), syntetc
‘ubreants and soWents for pals, They are also in cosmetics and are used as plastelsers in adhesWes and prtng inks.
Production and uses of glycol ethers and polyols
Ciyeol eters and polyols ae made fom epoxyethane by eacting wih alcohols:
Q
nic —‘CHy + ROH —* HO~(CH2CH.0})-R
Giycolethers produced wih n= 1 oF 2 and lower relatve molecular mass alechok are used as soWvents, Tivalnames are usually used for these
ebiers
Ethylone glycol monomethy| ether (methanol + 1 EO) and diethylene glycol monomethyl ether (metnanol-+ 2 EO) ae used as anteng addivos
Injettuets
\Where Ris fom a longer chain alcohol ang nis 3 or more, he products are known as ethoxylates. Alcohol ethoxyates, produced by reacting a
rq Cys leone!
with n=3 -10 molecules of EO, are widely used as nonionic surfactants (Imaterias-and-appleations/sutactans.himbinonionc) in allmanner of
cloning applications. Ris rom a aol oro, the resulting polyals are used to make polyurethanes (/polmersipolyurthane.him).
Production and uses of ethanolamines
Ethanolamines are produced trom epoxyethane by reacton wih ammonia
2.
a neni,
nH, —cH, + NH <——» "MN
ae t
venen ctenen
‘They are used in textle fishing, cosmetics, soaps, detergents, gas purifcation (as bases, they react wih, and remove, hydrogen sulfide, carbon
Gioxie and sulur dioxide) and corosion inhibitors.
Diethanolamine is used in a new greener method of manufacturing the herbciée, ayphosale (materal-and-applcationsierop-protaction-
choricas ntmitglyohosale) sol, or example, as "Roundup
Date last amended: 6h November 2016
Uhre
Aninvitation
We invite you to wite tous fyou have any specific comments about this ste, fr example
‘errors that you have found, suggestions for new topice or for adding to the existing unis,
suggestions for Inks to other ses and addons or alleratves to our examples.
Please send these comments to:
eci@essentatchemicalindusty.org(mailo:oci@essentalchemicalindusty org)
‘This web site is produced by the Centre for Industry Education Collaboration non-profit organization and an integral pat ofthe Department of
Chemistry, University of York UK. Copyright © 2016 Universit f York Cente for Industry Education Collaboration, York, UK. All Rights Reserve,
You might also like
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Selecting Screw CompressorsDocument3 pagesSelecting Screw Compressorsli xianNo ratings yet
- MAN Energy Compressors To Support Carbon Capture in NetherlandsDocument2 pagesMAN Energy Compressors To Support Carbon Capture in Netherlandsli xianNo ratings yet
- Heat Recovery Steam Generators: by TMI Staff & Contributors On February 16, 2020Document4 pagesHeat Recovery Steam Generators: by TMI Staff & Contributors On February 16, 2020li xianNo ratings yet
- Hydrogen PeroxideDocument5 pagesHydrogen Peroxideli xianNo ratings yet
- What Is Compressor SurgeDocument3 pagesWhat Is Compressor Surgeli xianNo ratings yet
- Ethane 1,2 Diol (Ethylene Glycol)Document4 pagesEthane 1,2 Diol (Ethylene Glycol)li xianNo ratings yet
- Catalysis in IndustryDocument10 pagesCatalysis in Industryli xianNo ratings yet
- SKF BearingDocument75 pagesSKF Bearingli xianNo ratings yet
- Air Separation MethodDocument11 pagesAir Separation Methodli xianNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)