Professional Documents
Culture Documents
Buta 1,3 Diene
Uploaded by
li xian0 ratings0% found this document useful (0 votes)
8 views2 pagesButa-1,3-diene process
Original Title
Buta-1,3-diene
Copyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentButa-1,3-diene process
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
8 views2 pagesButa 1,3 Diene
Uploaded by
li xianButa-1,3-diene process
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You are on page 1of 2
The Essential Chemical Industry - online
search
Buta-1,3-diene
Butasiene is manufactured from fractions obtained trom the distilation of ol. Athough by fr its major uses inthe HL CHa
‘manufacture of aii rubbers, some is used to make precursors in the manufacture of polyamides (nylon). aK,
Hac
Uses of butadiene
By far the greatest use of butadiene is in the manufacture of co-polymers produced ftom phenylethene (styrene) and butadiene, SBS
\ipolymersipolymers-an-overview himi#SBS_ABS) and propenontrie (acrylonitrile), butadiene and phenylethene (styrene), ABS
(ipolymersipolymers-an-overview:himlSBS_ABS). The nex’ biggest use isin the manufacture of poly(butadione), a synthetic rubber, which is
principally used as one of the components in the rubber used for car tyres:
Git HoLeH CHER} H,0=0:
Fne-oron—cnsh
‘toons
Other synthetic rubbers made from butadiene inclide neoprene whichis po(2-chlarobuta-2-diane, often known as pelychloraprene.
CChloraprene is made from butadiene, by frst reacting t wih chlorine in the gas phase at ca 500 Kto form 2-cichlorobutt-ene and #\4=
Aichlrobut2-ene, The former, on reaction wih sodium hydroxide, yields chloraprene:
HxC=CH— CH=CH,
| 00K
He=OH=CH=0h sean
& &
a segue sem
ad {chlorqprene)
on poymatn ep nos
a a a
» yc Tact
a o |,
scegueniagem nc
wanes aac tae,
Neoprene, in solutions an excellent adhesive and can also coat aluminium used in food packaging. is widely used in care, as hoses, vibration
‘mounts and shock
absorber seas is also used widely in constuction for example in window seals and in large constructions such as bridges.
Another eynthete co-polymer(polymersipalymere-an-overview:himi#SBS_ABS) made from butadiene is NBR (Nile Butadiene Rubber, te
other monomer being propenonitie (acryonitle) (polymersipolypropenonitile hn). NBR's abilly to withstand a range of temperatures from
220 to 380 K allows ibe used in several important aeronautical applicatons, for example to make fuel and othanding hoses and seas, where
ordinary rubbers cannot be used, Its also used to make protective gloves for use in te nuclear ndusby. NBRis also used in everyday toms,
for example in footwear
‘ater syne robert oer mala of utd ste mantacie of. -damnchexane(exametybnedamin) wich
‘made into a povamide Ypolymers/povamides him), Sesto vee
Diaminohexane fs made by passing hydrogen cyanide gas ito butadiene inthe quid state, under pressure at ca 350 K.
‘Anickelcompound is the catalyst forthe reaction. The overallreacton is represented by the equation:
H,O=CH-CHHCH) + 2HCeNa) “4M, WeC-cH CHCl CH, C=NO)
The resuling dnt s hydrogenated by passing #s vapour and hydrogen aver nickel at ca 00 K, under pressure (ca 35 atm)
Ng) + Hg) ESSE HN (CH,)y-NHAG)
NC (CHa)
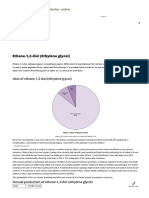
Annual production of butadiene
Wore 1055 mito tonnes!
[asia Pace mitlon omnes?
Nort Ameren | 3 milon omnes?
[weatem Europe] 2 mio tonnes?
Daf
1 Mach econ Coing 1. b 2. mne 207
2. lt Poco Onn Chg Ole Marit Nr 01 pa com risieinne ton
Manufacture of butadiene
Itis mainy manutactured by the
+ thermal cracking of naphtha oracesses/racking-isomeristion-and-retorming hi om oi
+ thermal cracking of butane Uprocesses/erackng-somerisation-and-reforming, Men (rm natural g9s and ol
Its leo made by he
+ thermal cracking of gas ol processesteraclngsomersation.and.teforming.hemh rom ll)
Date last amendeo: 25th April 2017
.
(repewitercorn
F urlehGaaess
repuinsen apse
Uhlan
31k
lane bender dent
‘Aninvitation
Wie invite you to wite tous fyou have any specific comments about this ste, for example
‘errors that you have found, suggestions for new topics or for adding to the existing unis,
‘suggestions for Inks to other ses and adadtions or alternatives to our examples.
Please send
eci@essentalchemicalindusty.org(mailo:oci@essentilchemicalindustry org)
‘comments to
‘Tis we ste s produced bythe Centre for Industy Eduction Callaberstion,anen-pot orangation and an itera pat fhe Deparment of
Chest, Universiy of York UK, Copy © 206 University York Cenze er Indsty Eduction Ceabration York UK. AN Rights Reser
You might also like
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Air Separation MethodDocument11 pagesAir Separation Methodli xianNo ratings yet
- SKF BearingDocument75 pagesSKF Bearingli xianNo ratings yet
- Selecting Screw CompressorsDocument3 pagesSelecting Screw Compressorsli xianNo ratings yet
- MAN Energy Compressors To Support Carbon Capture in NetherlandsDocument2 pagesMAN Energy Compressors To Support Carbon Capture in Netherlandsli xianNo ratings yet
- Heat Recovery Steam Generators: by TMI Staff & Contributors On February 16, 2020Document4 pagesHeat Recovery Steam Generators: by TMI Staff & Contributors On February 16, 2020li xianNo ratings yet
- What Is Compressor SurgeDocument3 pagesWhat Is Compressor Surgeli xianNo ratings yet
- Hydrogen PeroxideDocument5 pagesHydrogen Peroxideli xianNo ratings yet
- Catalysis in IndustryDocument10 pagesCatalysis in Industryli xianNo ratings yet
- Ethane 1,2 Diol (Ethylene Glycol)Document4 pagesEthane 1,2 Diol (Ethylene Glycol)li xianNo ratings yet