Professional Documents
Culture Documents
12 Chap 2 (Biological Molecules) F.SC 1st Year Biology Helping Notes PDF
Uploaded by
Zainab fatimaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
12 Chap 2 (Biological Molecules) F.SC 1st Year Biology Helping Notes PDF
Uploaded by
Zainab fatimaCopyright:
Available Formats
The College Study
Chap 2 (Biological Molecules) F.Sc 1st Year Biology Helping
Notes
Editorial Staff • March 26, 2019 8 minutes read
t/
ne
y.
ud
t
st
. n e
ge
t u le dy
es
ol
eg
ll
ec
co
he
.th
t
w
F.Sc Biology HSSC-I / 1st Year / 11th Class /
w
Part 1
//w
Chapter 2: Biological Molecules
s:
(Short Questions Answers)
tp
Cellulose is digested by the herbivores but not by humans. Why?
ht
In the herbivores (cow, buffalo etc.,) it is digested because of microorganisms (bacteria,
yeasts, protozoa) in their digestive tract. These microorganisms secrete an enzyme
called cellulase for its digestion. It is not digested in the human digestive tract as it lacks
cellulase secreting organisms.
What are lipids?
Appeared first @ www.thecollegestudy.net 1/10
The College Study
The lipids are a heterogeneous group of compounds related to fatty acids. They are
insoluble in water but soluble in organic solvents such as ether, alcohol, chloroform and
benzene. Lipids include fats, oils, waxes, cholesterol, and related compounds.
Why lipids store double the amount of energy as compared to the same
amount of any carbohydrate?
t/
Because of higher proportion of C-H bonds and very low proportion of oxygen, lipids
ne
store double the amount of energy as compared to the same amount of any
carbohydrate.
y.
What are different classes of lipids? OR classify lipids.
ud
Lipids have been classified as aclyglycerols, waxes; phospholipids. Sphingolipids,
t
glycolipids and terpenoid – lipids including carotenoids and steroids.
st
. n e
ge
dy
What are acylglycerols?
u
Chemically; acylglycerols can be defined as esters of fatty acids and alcohol.
t le
es
Acylglycerols are composed of glycerol and fatty acids. The most widely spread
ol
g
acylgycerol is triacyl glcerol, also called triglycerides or neutral lipids.
ll e
ec
o
What is an ester? Express it with an equation.
he c
.th
An ester is a compound produced as a result of a chemical reaction of an alcohol with
t
an acid and a water molecule is released.
w
C2H5OH + HOOCCH3 — > C2H5OCOCH3 + H2O
w
Differentiate between saturated and unsaturated fatty acids. Give examples.
//w
The fatty acids which contain no double bond are called saturated fatty acids e.g., acetic
acid, butyric acid, palmitic acid while those which have up to 6 double bonds are called
s:
unsaturated fatty acids e.g., Oleic Acid.
tp
How fatty acids of animals differ from those of plants?
ht
In animals the fatty acids are straight chains, while in plants these may be branched or
ringed. 59. Compare the solubility and melting points of palmitic and butyric acids. Ans:
Palmitic acid (C16) is much more soluble in organic solvent than butyric acid (CA). The
melting point of palmitic acid is 63.1°C as against -8°C for butyric acid.
Give examples of fibrous proteins.
Appeared first @ www.thecollegestudy.net 2/10
The College Study
Examples are silk fibre (from silk worm, and spiders web), myosin (in muscle cells) fibrin
(of blood clot), keratin (of nails and hair), and collagen (in connective tissues of skin,
bones, ligaments etc.).
Give the characters of globular proteins, or What are globular proteins?
These are spherical or ellipsoidal due to multiple folding of polypeptide chains.
t/
Tertiary structure is most important in them.
ne
They are soluble in aqueous media such as salt solution, solution of acids or bases,
or aqueous alcohol.
y.
They can be crystallized
ud
They disorganize with changes in the physical and physiological environment.
st
e
Give examples of globular proteins.
. n
ge
dy
Examples are enzymes, antibodies, hormones and haemoglobin.
From which cells nucleic acids were isolated?
t u le
es
ol
Nucleic acids were first isolated in 1870 by an Austrian physician Friedrich Miescher
eg
ll
from the nuclei of pus cells.
ec
co
Why nucleic acids are give that name?
he
.th
Due to their isolation from nuclei and their acidic nature, they were named re nucleic
t
w
acids.
w
How many types of nucleic acids are there?
//w
Nucleic acids are of two types, deoxyribonucleic acid or DNA and ribonucleic acid or
RNA.
s:
What is the effect of room temperature on fats?
tp
Fats containing unsaturated fatty acids are usually liquid at room temperature and are
ht
said to be oils. Fats containing saturated fatty acids are solids. Animal fats are solid at
room temperature, whereas most of the plant fats are liquid.
What is the specific gravity of fats and oils?
Fats and oils have a specific gravity of about 0.8.
Appeared first @ www.thecollegestudy.net 3/10
The College Study
What is the chemical nature or composition of waxes? or what are waxes?
Chemically, waxes are mixtures of long chain alkanes (with odd number of carbons
ranging from C25 to C35) and alcohols, ketones and esters of long chain fatty acids.
What is the chemical nature of phospholipids?
Phospholipids are derivatives of phosphatidic acid, which are composed of glycerol,
t/
fatty acids and phosphoric acid.
ne
Name the nitrogenous bases, which are important components of
y.
phospholipids.
Nitrogenous bases such as choline, ethanolamine and serine are important components
ud
of phospholipids.
st
Where are phospholipids found?
. n e
ge
dy
They are widespread in bacteria, animal and plant cells and are frequently associated
u
with membranes.
t le
es
ol
g
What are terpenoids? Give examples.
ll e
ec
Terpenoids are lipids which are made up of simple repeating units, isoprenoid. units.
o
This unit by condensation in different ways gives rise to compounds such as rubber,
he c
.th
carotenoids, steroids, terpenes etc.
t
w
What are the most abundant organic compounds to be found in cells?
w
Proteins are the most abundant organic compounds to be found in cells and comprise
over 50% of their total dry weight.
//w
What are proteins?
s:
Proteins are polymers of amino acids, the compounds containing carbon, nitrogen,
tp
oxygen and hydrogen.
ht
Give any two functions of proteins.
Carriers: Some proteins (e.g., hemoglobin) work as carriers and transport specific
substances such as oxygen, lipids, metal ions, etc.
Defend body: Some proteins, called antibodies, defend the body against pathogens.
What is the number of amino acids in proteins?
Appeared first @ www.thecollegestudy.net 4/10
The College Study
The number of amino acids varies from a few to 3000 or even more in different
proteins.
How many types of amino acids occur in cells and how many are found in
proteins?
About 170 types of amino acids occur in cells and tissues. Of these, about 25 are
t/
constituents of proteins. Most of the proteins are however made of 20 types of amino
ne
acids.
How RNA molecules occur?
y.
The RNA molecules occur as single strand, which may be folded back on to itself, to
ud
give double helical characteristics.
st
Whether RNA molecules occur as single strand or double strand?
. n e
ge
dy
The RNA molecules occur as single strand, which may be folded back on to itself, to
u
give double helical characteristics.
t le
es
ol
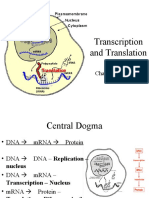
What is transcription?
eg
ll
It is the process by which RNA is synthesized from DNA.
ec
co
he
How many types of RNAs are there? or Name different types of RNA molecules.
.th
t
There are three types of RNA which are: messenger RNA (MRNA), transfer RNA (TRNA),
w
and ribosomal RNA (TRNA).
w
Where RNAs are synthesized?
//w
All the three types of RNAs are synthesized from DNA in the nucleus and then are
moved out in the cytoplasm to perform their specific functions.
s:
tp
What is the function of mRNA? or define mRNA?
It takes the genetic message from the DNA of the nucleus to the ribosomes in the
ht
cytoplasm to form particular proteins.
What is the length of mRNA?
mRNA consists of a single strand whose length depends upon the size of the gene as
well as the protein for which it is taking the message. For example for a protein
molecule of 1,000 amino acids, mRNA will have the length of 3,000 nucleotides.
Appeared first @ www.thecollegestudy.net 5/10
The College Study
What is the percentage of different types of RNAs in the cell?
mRNA is about 3 to 4% of the total RNA in the cell. tRNA comprises about 10 to 20% of
the cellular RNA. rRNA may be up to 80% of the total RNA.
What is the length of tRNA?
Transfer RNA molecules are small, each with a chain length of 75 to 90 nucleotides.
t/
ne
What is alpha carbon in amino acids?
All the amino acids have an amino group (-NH2) and a carboxyl group ( COOH)
y.
attached to the same carbon atom, also known as alpha carbon.
ud
What is the general formula of amino acids?
st
They have the general formula as R (amino group) H2N-CCOOH (carboxyl group) alpha
carbon.
. n e
ge
u
How głycylalanine is formed? or How peptide bond is formed?
t le dy
es
Glycylalanine is formed when amino acid glycine combines with amino acid alanine. The
ol
eg
linkage between the hydroxyl group of carboxyl group of one amino acid and the
ll
ec
hydrogen’ of amino group of another amino acid release H2O and C – N link to form
co
peptide bond. Thus a dipeptide, glycylalanine, is formed.
he
.th
t
How the specific properties of a protein are determined?
w
The specific properties of each protein are determined by the number and the specific
w
sequence of amino acids in a molecule, and also depend upon the shape which the
//w
molecule assumes as the chain folds into its final, compact form
What did F. Sanger conclude about insulin?
s:
Sanger concluded that insulin is composed of 51 amino acids in two chains. One of the
tp
chains had 21 amino acids and the other had 30. amino acids and they were held
ht
together by disulphide bridges
What is the composition of haemoglobin? or How many amino acid chains are
present in haemoglobin? Also give number of amino acids in each chain.
Haemoglobin is composed of four chains two alpha and two beta chains. Each alpha
chain contains 141 amino acids, while each beta chain contains 146 amino acids.
Appeared first @ www.thecollegestudy.net 6/10
The College Study
How the size of a protein is determined?
The size of a protein molecule is determined by the type of amino acids and the
number of amino acids comprising that particular protein molecule.
How many proteins are present in human body?
There are over 10,000 proteins in the human body which are composed of unique and
t/
specific arrangements of 20 types of amino acids.
ne
Why the arrangement of amino acids in a protein molecule is highly specific for
y.
its proper functioning.
It is because if any amino acid is not in its normal place, the protein fails to carry on its
ud
normal function. For example in the sickle cell haemoglobin only one of the 574 amino
t
acids do not occupy the normal place and the haemoglobin fails to carry any or
st
sufficient oxygen, hence leading to death of the patient.
. n e
ge
What are the two main types of secondary structure of proteins?
t u le dy
es
cc-helix: It involves a spiral formation of the basic polypeptide chain.
ol
eg
B-pleated sheet: It is formed by the folding back of the polypeptides.
ll
ec
o
How tertiary structure of protein is maintained?
he c
.th
It is maintained by three types of bonds, namely ionic, hydrogen, and disulfide (-S-S-).
t
w
Give an example of quaternary structure of protein.
w
Haemoglobin, the oxygen-carrying protein of red blood cells, has a quaternary
structure.
//w
Why it is very difficult to classify proteins?
s:
Due to complexity of structure and diversity in their function, it is very difficult to
tp
classify proteins.
ht
Classify proteins according to their structure.
Fibrous proteins
Globular proteins
Give the characters of fibrous proteins.
Appeared first @ www.thecollegestudy.net 7/10
The College Study
They consist of molecules having one or more polypeptide chains in the joint form
of fibrils. Secondary structure is most important in them.
They are insoluble in water
They are non-crystalline
They are elastic in nature.
t/
Where does DNA occur in cell?
ne
DNA occurs in chromosomes, in the nuclei of the cells and in much lesser amounts in
mitochondria and chloroplasts.
y.
ud
Where RNA is present in the cell?
RNA is present in the nucleolus, in the ribosomes, in the cytosol and in smaller amounts
st
e
in other parts of the cells..
. n
ge
dy
What are nucleic acids? Or Nucleic acids are polymers of units of which
u
component?
t le
es
The nucleic acids are polymers of units called nucleotides. DNA is made up of
ol
eg
deoxyribonucleotides, while RNA is composed of ribonucleotides.
ll
ec
o
What are the subunits of a typical nucleotide?
c
he
.th
Each nucleotide is made of three subunits:
t
w
a 5-carbon monosaccharide (a pentose sugar)
w
a nitrogen-containing base
//w
a phosphoric acid
What pentose sugar is found in nucleotides? (OR How ribonucleotide and
s:
deoxyribonucleotide differ in having pentose sugar?)
tp
Pentose sugar in ribonucleotide is ribose, while in deoxyribonucleotide it is deoxyribose
ht
(one oxygen removed from OH group at carbon number
What nitrogenous bases are present in nucleic acids?
Nitrogenous bases are of two types:
Appeared first @ www.thecollegestudy.net 8/10
The College Study
single-ringed pyrimidines
double-ringed purines
Pyrimidines are cytosine (abbreviated as C), Thymine (abbreviated as T), and uracil (U).
Purines are adenine (A) and guanine (G).
t/
What is the position of attachment of nitrogenous base and phosphoric acid to
pentose sugar in a typical nucleotide?
ne
In a typical nucleotide the nitrogenous base is attached to position 1 of pentose sugar,
y.
while phosphoric acid is attached to carbon at position 3 of pentose sugar.
ud
Differentiate between nucleoside and nucleotide.
t
The compound formed by combination of a base and a pentose sugar is called
st
. n e
nucleoside while a nucleoside and a phosphoric acid combine to form a nucleotide.
ge
Name a nucleotide used as an energy currency by the cell.
le
t u dy
es
ATP is the nucleotide used as an energy currency by the cell.
ol
eg
ll
What kinds of nucleotides DNA is made up of?
ec
o
It is made of four kinds of nucleotides namely, d-adenosine monophosphate (d-AMP,
he c
.th
adenylic acid), d-guanosine monophosphate (d-GMP, also known as d-guanylic acid); d-
t
Cytidine monophosphate (d-CMP, also known as de cytidylic acid) and d-thymidine
w
monophosphate (d-TMP, also known as d thymidylic acid).
w
What is polynucleotide chain?
//w
The nucleotides are united with one another through phosphodiester linkages in a
specific sequence to form long chains known as polynucleotide chains.
s:
What is NAD?
tp
Nicotinamide adenine dinucleotide, abbreviated as NAD, is an example of dinucleotide.
ht
It is an important coenzyme in several oxidation-reduction reactions in the cells.
What is the amount of DNA in a cell?
The amount of DNA is fixed for a particular species, as it depends upon the number of
chromosomes. The amount of DNA in germ cells (sperms and ova) is one half to that of
somatic cells (skin, muscle, bone cells etc.)
Appeared first @ www.thecollegestudy.net 9/10
The College Study
Check also : F.Sc 1st Year (Biology) Helping Notes
You can get PDF Copy of this article for offline purposes by using above
t/
PDF Download option.
ne
y.
ud
t
st
. n e
ge
t u le dy
es
ol
eg
ll
ec
co
he
.th
t
w
w
//w
s:
tp
ht
Appeared first @ www.thecollegestudy.net 10/10
You might also like
- Anatomy and Physiology for Students: A College Level Study Guide for Life Science and Allied Health MajorsFrom EverandAnatomy and Physiology for Students: A College Level Study Guide for Life Science and Allied Health MajorsNo ratings yet
- Chapter 05Document12 pagesChapter 05homamunfatNo ratings yet
- Biology NotesDocument60 pagesBiology Noteslogan hNo ratings yet
- Biochemistry Past Paper QuestionDocument20 pagesBiochemistry Past Paper QuestionAchraf RabadiNo ratings yet
- F.SC (1st Year) Biology Chapter Wise Short Questions AnswersDocument10 pagesF.SC (1st Year) Biology Chapter Wise Short Questions AnswersAyoob Rana67% (3)
- Lipids 2Document44 pagesLipids 2zubair saeedNo ratings yet
- Chemistry (Part 2) Chapter 14. Macromolecules Short QuestionsDocument9 pagesChemistry (Part 2) Chapter 14. Macromolecules Short QuestionsMajid HafeezNo ratings yet
- Lipids Chapter SummaryDocument18 pagesLipids Chapter SummaryBizuayehu Ze GeorgeNo ratings yet
- Fatty Acid MetabolismDocument8 pagesFatty Acid MetabolismJessicaOktavianusNo ratings yet
- Botany XI STBBDocument21 pagesBotany XI STBBHabib U Zaman MemonNo ratings yet
- Physical Science Handout Week5-6Document19 pagesPhysical Science Handout Week5-6Jhay Lorraine Sadian PalacpacNo ratings yet
- Biological Molecules 10BDocument41 pagesBiological Molecules 10BShamshad ShaheerNo ratings yet
- Biological Molecules: The Building Blocks of LifeDocument66 pagesBiological Molecules: The Building Blocks of Life:Y FrankNo ratings yet
- Chap.1-4Introduction To General BiologyDocument95 pagesChap.1-4Introduction To General BiologyThomas Hika100% (1)
- Lipids Classification GuideDocument28 pagesLipids Classification GuideX x A7md x XNo ratings yet
- Biology Form 4 LipidsDocument14 pagesBiology Form 4 LipidsSofea RamieNo ratings yet
- 08LipAMO SuarezDocument15 pages08LipAMO SuarezscasuarezNo ratings yet
- Chapter Six, GeneticsDocument10 pagesChapter Six, GeneticsHashim GhazoNo ratings yet
- Chapter 9 Biomolecules 9.1 How To Analyse Chemical Composition?Document8 pagesChapter 9 Biomolecules 9.1 How To Analyse Chemical Composition?Charin KadianNo ratings yet
- EDEXCEL: AS BIOLOGY REVISION NOTES: UNIT 1 METABOLISM OVERVIEWDocument14 pagesEDEXCEL: AS BIOLOGY REVISION NOTES: UNIT 1 METABOLISM OVERVIEWAe Banpong0% (2)
- LipidsDocument4 pagesLipidsAshley ArnoldNo ratings yet
- Biological Molecules 0Document12 pagesBiological Molecules 0rhyshryroch royerasNo ratings yet
- Lipids (S) PDFDocument5 pagesLipids (S) PDFMathura MathuNo ratings yet
- Biology Notes: Bell High SchoolDocument42 pagesBiology Notes: Bell High SchoolRichard Ye93% (14)
- Key Concepts: BiochemistryDocument24 pagesKey Concepts: BiochemistryJustyNo ratings yet
- Membrane LipidsDocument42 pagesMembrane LipidsStanley ChikoveNo ratings yet
- Lipids ExplainedDocument20 pagesLipids ExplainedARYAN TIWARINo ratings yet
- Biological MacroDocument49 pagesBiological MacroChristine De San JoseNo ratings yet
- BIOMOLECULES NOTE 11aaDocument9 pagesBIOMOLECULES NOTE 11aaGyaniNo ratings yet
- BIOLOGY M4 SlideDocument18 pagesBIOLOGY M4 SlideMohammad Ammar Mohamed YassinNo ratings yet
- Biomolecules (Carbohydrates, Fats, Proteins and Nucleic Acids)Document4 pagesBiomolecules (Carbohydrates, Fats, Proteins and Nucleic Acids)rommel benamirNo ratings yet
- Lipids: Classification, Structure and FunctionsDocument9 pagesLipids: Classification, Structure and FunctionsJade PategaNo ratings yet
- The Structures, Properties, and Functions of BiomoleculesDocument49 pagesThe Structures, Properties, and Functions of BiomoleculesKirsten Andrea MagallanesNo ratings yet
- CNP 3 LipidsDocument49 pagesCNP 3 LipidsKepa KepaNo ratings yet
- Lipid NotesDocument5 pagesLipid NotesGary TeohNo ratings yet
- The Lipids: Triglycerides, Phospholipids, and Sterols: Learning ObjectivesDocument9 pagesThe Lipids: Triglycerides, Phospholipids, and Sterols: Learning Objectiveslianghoo94No ratings yet
- Living OrganizationDocument10 pagesLiving OrganizationAbbas TalibNo ratings yet
- Mader/Biology, 11/e - Chapter Outline: 3.1 Organic MoleculesDocument6 pagesMader/Biology, 11/e - Chapter Outline: 3.1 Organic Moleculesapi-455371000No ratings yet
- Physical Science Lesson 13 The Structure and Properties of MatterDocument17 pagesPhysical Science Lesson 13 The Structure and Properties of MatterJustin BirdNo ratings yet
- Organic Molecules: The Chemistry of CarbonDocument7 pagesOrganic Molecules: The Chemistry of CarbonPangkat DalawaNo ratings yet
- Chemistry and Digestion of LipidsDocument35 pagesChemistry and Digestion of LipidsBiph BiphNo ratings yet
- Biology Notes Pages 2Document81 pagesBiology Notes Pages 2Quynh NhuNo ratings yet
- Mrs. Tarawally - 045400Document11 pagesMrs. Tarawally - 045400koromamoses235No ratings yet
- Lesson 1 Introduction To BiochemistryDocument7 pagesLesson 1 Introduction To BiochemistryMAN'S BEST FRIENDNo ratings yet
- Grade 10 4th Quarter Week 3Document12 pagesGrade 10 4th Quarter Week 3KarenNo ratings yet
- BiomoleculesDocument11 pagesBiomoleculesHarrish SandeevNo ratings yet
- Biological Molecules: The Building Blocks of LifeDocument114 pagesBiological Molecules: The Building Blocks of Lifeカガミネ りNo ratings yet
- Carbohydrates Lipids Proteins Nucleic AcidsDocument14 pagesCarbohydrates Lipids Proteins Nucleic AcidsRenee RoSeNo ratings yet
- Ethiopia Institute of Biotechnology: Table: A Comparison of Elements Present in Non-Living and Living MatterDocument25 pagesEthiopia Institute of Biotechnology: Table: A Comparison of Elements Present in Non-Living and Living Mattergebremeskel hagosNo ratings yet
- Crash Course KEY NOTES on BiomoleculesDocument6 pagesCrash Course KEY NOTES on BiomoleculesDebkanta Roy0% (1)
- Cytolgy 2Document7 pagesCytolgy 2Mohammad RajabNo ratings yet
- Lipids HoDocument9 pagesLipids HoDominic JoseNo ratings yet
- 87314094-83be-428d-9d4d-a459e43691a9Document21 pages87314094-83be-428d-9d4d-a459e43691a9SAMPATH SPNo ratings yet
- BCH301 Introduction to Biochemistry Chapter 6: LipidsDocument55 pagesBCH301 Introduction to Biochemistry Chapter 6: Lipidsreem aldanafNo ratings yet
- Notes Biological Molecules 3Document8 pagesNotes Biological Molecules 3abdul rehmanNo ratings yet
- Lipids Lect (1) الدهون كمياء حياتيةDocument6 pagesLipids Lect (1) الدهون كمياء حياتيةuuuhbnb lplhghNo ratings yet
- Are Unhe Althy, E.: "The Hidd Enk Iller"Document63 pagesAre Unhe Althy, E.: "The Hidd Enk Iller"Math YehualaNo ratings yet
- Che 473 Presented by - Nishatanjum (0802005) Seefat Farzin (0802011) Turna Barua (0802016)Document30 pagesChe 473 Presented by - Nishatanjum (0802005) Seefat Farzin (0802011) Turna Barua (0802016)asha196No ratings yet
- Lipids and MembranesDocument9 pagesLipids and MembranesPatricia Bianca BunagNo ratings yet
- Role of Lipids in The BodyDocument16 pagesRole of Lipids in The BodyKaila SalemNo ratings yet
- General BiologyDocument19 pagesGeneral BiologyHannah RodriguezNo ratings yet
- Carbohydrate Metabolism 1Document70 pagesCarbohydrate Metabolism 1nia adelleNo ratings yet
- (Doi 10.1002 - 9780470015902.a0000498.pub2) Teillaud, Jean-Luc - ELS - Antibody-Dependent Cellular Cytotoxicity (ADCC)Document8 pages(Doi 10.1002 - 9780470015902.a0000498.pub2) Teillaud, Jean-Luc - ELS - Antibody-Dependent Cellular Cytotoxicity (ADCC)SyahputraWibowoNo ratings yet
- Fatty Acid Beta OxidationDocument59 pagesFatty Acid Beta OxidationEve YapNo ratings yet
- Commentary With Professor Serge Mostowy and Artist Samantha Moore On A Language of Shapes'Document4 pagesCommentary With Professor Serge Mostowy and Artist Samantha Moore On A Language of Shapes'Animate ProjectsNo ratings yet
- Immune System Review QuestionsDocument2 pagesImmune System Review Questionsapi-521773978No ratings yet
- Earth and Life Science SHS 16.1 Cell The Basic Unit of LifeDocument18 pagesEarth and Life Science SHS 16.1 Cell The Basic Unit of LifeWinsear VardeNo ratings yet
- Class 11 Cell Cycle and Cell DivisionDocument2 pagesClass 11 Cell Cycle and Cell Divisionjeeneet.alwalNo ratings yet
- KERATOKONUSDocument15 pagesKERATOKONUSLusyAlwiNo ratings yet
- Proteins Classification GuideDocument7 pagesProteins Classification GuideKrishnanand NagarajanNo ratings yet
- AP Bio CH 14 Gene ExpressionDocument24 pagesAP Bio CH 14 Gene ExpressionLuisa RamosNo ratings yet
- Regulation of Gene Expression From Lehninger - 4e PDFDocument21 pagesRegulation of Gene Expression From Lehninger - 4e PDFAlthea Karmylle M. BonitaNo ratings yet
- 12 - Energy - and - Respiration 9700Document6 pages12 - Energy - and - Respiration 9700Rajesh KumarNo ratings yet
- Nucleic Acids and Overview of Central Dogma - SAC - Janine Teza S. VillenaDocument52 pagesNucleic Acids and Overview of Central Dogma - SAC - Janine Teza S. VillenaNovie Carla GayosaNo ratings yet
- Investigating The Efficacy of Nisin When Combined With First Line Antibiotics in The Treatment of MRSA 252Document47 pagesInvestigating The Efficacy of Nisin When Combined With First Line Antibiotics in The Treatment of MRSA 252Joe CloNo ratings yet
- Crop Science Notes 2 2015Document67 pagesCrop Science Notes 2 2015Alfred Magwede100% (1)
- Test Bank For Principles of Biochemistry 4th Edition HortonDocument12 pagesTest Bank For Principles of Biochemistry 4th Edition Hortonhightpiprall3cb2No ratings yet
- Beta Oxidation of Fatty AcidsDocument4 pagesBeta Oxidation of Fatty AcidsJonas Galeos0% (1)
- Act05 Animal Histology and Organology DiscussionDocument9 pagesAct05 Animal Histology and Organology DiscussionPaula Nicole AlanoNo ratings yet
- Solutions MidtermDocument3 pagesSolutions MidtermAli NajmaldinNo ratings yet
- MLS 047 Molecular Biology and Diagnostics Student Activity SheetDocument10 pagesMLS 047 Molecular Biology and Diagnostics Student Activity SheetYlia MastarsNo ratings yet
- 1.1 Extraction and Characterization of Proteins 01Document9 pages1.1 Extraction and Characterization of Proteins 01Michelle YapNo ratings yet
- RMC-0708 Drives Tumor Regressions in KRASQ61H ModelsDocument1 pageRMC-0708 Drives Tumor Regressions in KRASQ61H Models肖茹雪No ratings yet
- Mitochondria HeartDocument2 pagesMitochondria HeartSrinivas PingaliNo ratings yet
- Biology Edexcel A2 Unit 5 NotesDocument2 pagesBiology Edexcel A2 Unit 5 NotesAbigail Falola100% (2)
- Human Molecular Genetics, Fourth Edition True PDF (PDFDrive) PDFDocument812 pagesHuman Molecular Genetics, Fourth Edition True PDF (PDFDrive) PDFPasquale Vitale33% (3)
- Protein SynthesisDocument29 pagesProtein SynthesisEugenia Migranova100% (9)
- BIOL 2050 - Lecture 7Document27 pagesBIOL 2050 - Lecture 7WavyBaconNo ratings yet
- Government College University, Faisalabad: Max Marks 32 (20+12)Document2 pagesGovernment College University, Faisalabad: Max Marks 32 (20+12)Arslan Muhammad EjazNo ratings yet
- Cell Membrane Bubble LabDocument7 pagesCell Membrane Bubble LabRic Anthony LayasanNo ratings yet