Professional Documents
Culture Documents
US2497309
Uploaded by
xcvCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
US2497309
Uploaded by
xcvCopyright:
Available Formats
Feb.
14, 1950 A, T, LARSON ETA 2,497,309
PROCESS FOR THE PREPARATION OF ETHYLENEUREA
Filed May 4, 1944
D
WA7AAR
AA/MOWAA. cowVEerzR 2
AIAYZFME 6. N
DAMNSS/OAGE S7R/PAIW6
Nae O
st
D)
coa -> -s; 4. S1
- B
AACAF//AAR
a 7. ) |\i.
e ZO
4&fzeo/7. A62/SO72 newors
A 727/2Zz72 G 1a/e72
BY
WAY
Patented Feb. 14, 1950 2,497,309
UNITED STATES PATENT OFFICE 2,497,309
PROCESS FOR THE PREPARATION OF
ETHYLENEUREA
Alfred T. Larson and Arthur G. Weber, Wilming
ton, Del, assignors to E. I. du Pont de Nemours
& Company, Wilmington, Del, a corporation
of Delaware
Application May 4, 1944, Serial No. 534,046
11. Claims. (CI. 260-309)
2
This invention relates to a process for the prep ranging from about 175° C. to about 300° C. At
aration of N.Nethyleneurea, and more particu temperatures approaching 300° C., however, the
larly to its preparation from ethylenediamine and crude product is somewhat darkened in color, in
carbon dioxide. The subject matter of this case dicating some decomposition, and this may be
is related to the A. T. Larson copending applica- . avoided by conducting the reaction at tempera
tions S. N. 524,525, which has become abandoned, tures somewhat below 300° C., say, for example,
and S.N. 524,527, filed March 1, 1944. below 270° C. but above 75° C. Elevated pres
N.Nethyleneurea, hereinafter referred to as sures may be used and may range from 10 or 500
ethyleneurea, and otherwise known as 2-oxo atmospheres or more.
imidazolidin and imidazolidon (2), has been made () The ratio of ethylenediamine to carbon dioxide
by heating ethylenediamine with diethyl carbon may vary over a wide range. For example, the
ate at 180° C. E. Fisher, Koch, A 232, 227 carbon dioxide may be present in excess, thereby
(1886), by Warming an aqueous solution of giving acid reaction conditions and more or leSS
N.Nethylene thiourea, with freshly precipitated solid reactants or the ethylenediamine may be
mercuric oxide Klut Ar. 240, 677 (1887), and by 5 present in excess giving a slurry Or homogenous
distillation of aqueous N.Nethylene guanidine solution. Accordingly, there may be present from
under diminished pressure Pierron A 9 (11) 0.1 to 15 moles of carbon dioxide per mole of
363 (1908). Ethyleneurea has remained more ethylenedianine on a weight basis, although an
or less a laboratory curiosity, however, for the excess of ethylenediamine is preferably used in
above processes to date have been of only aca the order of about 1,01 to 1.5 moles thereof per
demic significance. mole of carbon dioxide.
An object of the present invention is to provide The reaction will produce ethyleneurea in the
an improved process for the preparation of ethyl presence or absence of Water, but for best results
eneurea. Another object of the invention is to aqueous solutions of ethylenediamine are used.
provide a process for the preparation of ethylene 25 The azeotrope of ethylenediamine and water
urea, from ethylenediamine and carbon dioxide. (84% diamine, 16% water) or other suitable aque
Yet another object is to provide a process for the Oussolutions of ethylenediamine containing from
preparation of ethyleneurea from ethylenedi 10 to 50% water appear to be as effective as the
amine, water and carbon dioxide under elevated anhydrous compound.
temperatures. A further object is to provide a 30 crude The ethyleneurea, may be separated from the
continuous process for the preparation of ethyl reaction mixture by evaporation, by vac
eneurea, from mixtures of ethylenediamine and uum distillation or by steam distillation, i. e. by
carbon dioxide under elevated temperatures and heating the mixture to distillation temperature
pressures. Other objects and advantages of the while passing steam through it. Alternatively,
invention will hereinafter appear. 35 the crude product may be subjected to crystalliza
Broadly, the process of the invention may be tion for the separation of ethyleneurea, the crys
tallization being conducted in a suitable solvent
described as involving the high temperature re
action of ethylenediamine with carbon dioxide. therefor.
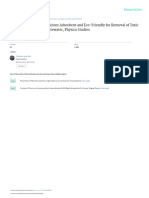
For example, a pressure resisting reaction vessel The process is carried out by passing ethylene
diamine, preferably as an aqueous solution, and
is continuously charged with anhydrous or aque 40 carbon
Ous ethylenediamine and carbon dioxide. It has dioxide continuously into a tubular type
been determined that carbon dioxide reacts with reactor. The continuous operation of the piocess
ethylenediamine to first give an ethylenediamine is illustrated by the attached drawing wherein
carbamate which may be formed as a solution, carbon dioxide through pipe A and ethylenedi
slurry or solid according to the proportions of re 45 amine from storage tank B are introduced into
actants, an excess of the ethylenediamine giving a silver-lined preheater wherein the reaction
a solution or slurry, while an excess of carbon mixture is raised to approximately reaction tenn
dioxide produces a solid. If the reaction is con perature and from this preheater the mixture is
ducted rapidly, which is possible if it is carried introduced into the converter 2 which consists of
out continuously, the carbamate appears to have 50 a silver-lined reaction tube of relatively great
but a transient existence. In any event, by con length to diameter. In this tube the reaction
ducting the process continuously and heating to mixture is maintained at a temperature above
temperatures described below, the conversion of 175 C. and a pressure above 10 atmospheres. If
the ethylenediamine to ethyleneurea is assured. Carbon dioxide is in excess of the stoichiometric
The reaction is conducted at temperatures 55 requirements the operating pressure is preferably
2,497,309
3 4
above 100 atmospheres. The reaction mixture ture bath, raised to a temperature of 225 C. and
issuing from converter 2 is passed into the water shaken throughout the reaction to mix thorough
removal column 3, heated by calandria, 3a, ly the reactants. During the reaction, which was
Wherein a major portion of the Water is distiled continued at temperature for about 90 minutes,
from the unconverted ethylenedianine. The a pressure of 625 atmospheres was developed.
crude reaction mixture is then passed through The autoclave was cooled, its pressure released
heater 4 wherein the ethylenedianine is vaporized and the product evaporated to dryness to give
at a temperature of about 170° C. and the result about an 87% yield of ethylene diamine to ethyl
ing liquid vapor mixture treated in separator 5, eele,
the Vapors being passed to condenser f, the liq O Eacample 3
luids to stripping column 6. The separator 5 may
be a cyclone separator in which the entering A silver-lined pressure resisting autoclave was
liquid-vapor mixture is introduced tangentially charged. With 0.5 mole of ethylene diamine, 0.8
to the inner cylindrical upper wall. The liquid mole of Water and 5.0 moles of carbon dioxide.
containing ethyleneurea, is treated in stripping 5 The autoclave Was placed in a constant tempera
Column 6 to remove the last of the water, CO2 and ture bath, raised to a temperature of 250 C. and
ethylenediamine by countercurrent scrubbing shaken throughout the reaction to mix thorough
with annonia (nitrogen, methane, carbon mon ly the reactants. During the reaction, which was
Oxide, or other inert gas) at a temperature of ap continued at temperature for about 35 minutes,
proximately 170° C. The scrubbing medium a pressure of 795 atmospheres was developed,
passes through the stripping column condenser The autoclave Was cooled, its pressure released
and separator 6a, and pump 6b back to the column and the product evaporated to dryness to give
6. The water, ethylendiamine and CO2 are re about an 80% yield of ethylene diamine to ethyl
turned to the storage tank B. The stripping col eerea,
unn 6 is preferably a packed column. From the 25 Eacample 4
bottom of the stripping column 6 the ethylene
urea is dicharged to the ethyleneurea, still oper 1% moles of ethylenediamine (containing 32%
ated under a vacuum from which it is distilled Water) Was pumped from the storage tank to a
into the receiver 8 from which it passes directly tee where it was mixed with one mole of carbon
to the flaker roll 9 which consists of a chromium 30 dioxide and introduced continuously into pre
plated cylindrical roll cooled to a temperature of heater . In preheater the temperature of the
approximately 30° C. upon which the ethylene mixture was raised to approximately 200° C. and
urea, Solidifies and is scraped off in a fake-like the mixture then passed into converter 2. This
form into the hopper O. The ethylenedianine, converter consisted of a silver-lined tubular re
CO2 and water from cyclone separator 5 is passed 35 action zone of s' I. D. and 75 feet in length.
to condenser , recycled to the system and re The reaction was conducted in converter 2 at a
introduced with make-up ethylenediamine and preSSure of about 70 atmospheres, a temperature
carbon dioxide into the preheater . of about 250° C. with a time of reaction of from
Alternatively, the process may be carried out by 12 to 15 minues. The product was continuously
preheating the ethylenediamine and carbon di 40 discharged from the converter into the water re
Oxide separately, mixing them only as they are in moVal still 3 in which the Water was removed at
troduced at approximately reaction temperature a temperature of about 212° C. The substantially
into the reaction zone. This procedure eliminates Water-free crude product was then passed
the possibility of the formation of large amounts through the heater 4 wherein it was heated to a
of ethylenediamine carbamate. temperature of about 170° C. and into the cyclone
In lieu of water as a solvent for the reaction separator 5 in which most of the ethylenediamine
other inert liquids may be used such as the and Water Vapors were separated from the ethyl
higher boiling alcohols, e. g. propanol, butanol eneurea, and the semi-purified ethyleneurea, dis
or cyclohexanol; the ethers, e. g. dibutyl ether, charged into the stripper column 6 wherein the
dioxane or the cyclic ethers, and such diluent 50 final traces of Water and unconverted ethylenedi
Which may or may not give miscible mixtures, annine Were driven off by contact with a counter
e.g. benzene, toluene, etc. Current flow of nitrogen at a temperature of
The examples illustrate preferred embodiments about 170° C. From stripping column 6 the prod
of the invention wherein parts are by weight un uct was dropped into the still maintained at
less otherwise indicated. 55 about 20 mm. pressure and a temperature of
Eacample 1 about 200° C. and the distillate passed into con
A silver-lined pressure resisting autoclave was denser 8, the ethyleneurea was condensed and
charged with 0.5 mole of ethylene diamine, 0.8 passed to the receiver 8a and faker roll 9. The
Inole of Water and 5.0 moles of carbon dioxide. final product upon analysis revealed an ethylene
The autoclave was placed in a constant tempera
60 diamine Conversion per pass of about 55% with an
ture bath, raised to a temperature of 250° C. and Overal yield of about 88%.
shaken throughout the reaction to mix thorough We cairn:
ly the reactants. During the reaction, which was 1. A continuous cyclical process, for the prepa
Continued at temperature for about 60 minutes, ration of ethyleneurea, which comprises continu
a pressure of 860 atmospheres was developed. 65 ously introducing ethylenediamine, containing
The autoclave Was cooled, its pressure released from 10 to 50% water, and carbon dioxide into
and the product evaporated to dryness to give a reaction zone, subjecting the resulting mixture
about a 70% yield of ethylene diamine to ethyl therein to a temperature between 200 and 300° C.
eneurea. and a pressure above 10 atmospheres, continuous
Eacample 2 70 ly discharging the products from the reaction
Zone, separating the ethyleneurea from the water,
A silver-lined pressure resisting autoclave was
charged with 0.51 mole of ethylene diamine, 0.8 the unreacted ethylenediamine and carbon di
Oxide and returning the unreacted ethylenedi
mole of Water and 5.0 moles of carbon dioxide. amine and carbon dioxide, adding more carbon
The autoclave was placed in a constant tempera 75 dioxide and the amount of ethylenediamine con
2,497,309
5 6
Sumed in the cycle thereto and passing the re 7. In a continuous process for the preparation
Sulting mixture into the reaction Zone. of ethyleneurea by subjecting ethylenediamine
2. A continuous process for the preparation and carbon dioxide to a reaction at a temperature
of ethyleneurea, which comprises continuously in between 170 and 300° C. and under a pressure of
troducing ethylenediamine containing from 10 to at least 10 atmospheres, the steps which com
50% water and carbon dioxide into a reaction prise subjecting the reaction mixture containing
Zone of relatively great length to diameter, sub ethyleneurea, unconverted ethylenediamine, car
jecting the resulting mixture therein to a tem bon dioxide and water to distillation for the re
perature between 200 and 300° C. and a pressure moval of a major portion of the water, to a flash
above 10 atmospheres, continuously discharging vaporization for the removal of a major portion
the products from the reaction Zone, separating of the unconverted ethylenediamine, to a stripper
the ethyleneurea, from the unreacted ethylenedi action with an inert gas to remove by vaporiza
amine and carbon dioxide and returning the eth tion remaining unreacted ethylenediamine and
ylenediamine and carbon dioxide to the reaction Water and subsequently purifying the ethylene
2Ole. 5 urea by vacuum distillation.
3. A continuous process for the preparation of 8. In a continuous process for the preparation
ethyleneurea, which comprises continuously intro of ethyleneurea, by subjecting ethylenediamine
ducing ethylenediamine, water and carbon dioxide and carbon dioxide to a reaction under pressures
into a reaction Zone of relatively great length to above 10 atmospheres and temperatures between
diameter, subjecting the resulting mixture there 200 and 300° C. the step which comprises con
in to a temperature between 200 and 300° C. and tinuously introducing carbon dioxide and ethyl
a pressure above 10 atmospheres, continuously enediamine directly into the reaction zone.
discharging the products from the reaction Zone, 9. The process in accord with claim 8 in which
separating the ethyleneurea from Water, carbon the ethylenediamine and water are each separate
dioxide and the unreacted ethylenediamine and ly preheated prior to introducing them into the
returning the ethylenediamine to the reaction reaction Zone.
2Ole. 10. The process in accord with claim 8 in which
4. In a continuous process for the preparation the ethylenediamine and water are each separate
of ethyleneurea by subjecting ethylenediamine ly preheated at 200° C. prior to introducing them
and carbon dioxide to reaction under preSSures into the reaction zone.
above at least 10 atmospheres arid a temperature 11. A process for the preparation of ethylene
above 170° C. but below the temperature at which urea, which comprises subjecting an aqueous solu
ethyleneurea, is decomposed, the steps which com tion of ethylenediamine to a reaction with carbon
prise continuously passing the reaction products dioxide at a temperature between 175° C. but
into a heating zone from which the products 3 below the temperature at which ethyleneurea de
pass as a two phase liquid-vapor mixture and composes, under a pressure above 10 atmospheres,
continuously separating the liquid from the the ethylenediamine being present in excess on
vapor. a molecular weight basis.
5. In a continuous process for the preparation
of ethyleneurea by subjecting ethylenediamine 40 ALFRED T. LARSON.
and carbon dioxide to reaction under pressures ARTHUR. G. WEBER,
above at least 10 atmospheres and a temperature REFERENCES CITED
above 170° C. but below the temperature at which
ethyleneurea is decomposed, the steps which con The following references are of record in the
prise continuously passing the reaction products 45 file of this patent:
into a heating Zone from which the products paSS UNITED STATES PATENTS
as a two phase liquid-vapor mixture, continuous
ly separating the liquid from the vapor, Con Number Name Date
densing and recycling part of the vapors, sub 1,307,570 Wenkel ------------ June 24, 1919
jecting the liquid to a stripping operation for 50 1,785,730 Davis ------------- Dec. 23, 1930
the removal of residual ethylenediamine and 1816,087 Lindner et al. ------ July 28, 1931
water vapor and finally recovering the ethylene 2,087,325 Lawrence et al. ---- July 20, 1937
ea. 2,194,082 Booth ------------- Mar. 19, 1940
6. In a continuous process for the preparation 2,212,847 Porter ------------ Aug. 27, 1940
of ethyleneurea by subjecting ethylenediamine 55 2,276,696 Olin -------------- Mar. 17, 1942
and carbon dioxide to reaction under preSSures FOREIGN PATENTS
above at least 10 atmospheres and a temperature
above 170° C. but below the temperature at which Nube Country Date
ethyleneurea is decomposed, the steps which com 123,138 Germany --------- July 30, 1901
prise continuously passing the reaction products 80 OTHER REFERENCES
into a heating Zone from which the products pass,
the unconverted ethylenediamine, carbon dioxide Fischer-Koch, Annalen, vol. 232, p. 227 (1886).
and water in the vapor phase and the ethylene Chem, Abstracts, vol. 32, p. 488,
urea, in the liquid phase, and cyclonically separat
ing the two phases.
You might also like
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Prep 201300063Document10 pagesPrep 201300063xcvNo ratings yet
- US2945890 2,4-DinitroresorcinolDocument1 pageUS2945890 2,4-DinitroresorcinolxcvNo ratings yet
- Exploders Google Translate AgN3Document1 pageExploders Google Translate AgN3xcvNo ratings yet
- Benedikt and Hubl JCS 1881Document4 pagesBenedikt and Hubl JCS 1881xcvNo ratings yet
- Diazidodinitrohydroquinone H-228 From Vol. 7 H-LDocument1 pageDiazidodinitrohydroquinone H-228 From Vol. 7 H-LxcvNo ratings yet
- 1,4-Diformyl-2,3,5,6-TetranitratopiperazineA New Primary Explosive Based On GlyoxalDocument6 pages1,4-Diformyl-2,3,5,6-TetranitratopiperazineA New Primary Explosive Based On GlyoxalxcvNo ratings yet
- Diazidodinitrohydroquinone H-228 From Vol. 7 H-LDocument1 pageDiazidodinitrohydroquinone H-228 From Vol. 7 H-LxcvNo ratings yet
- A New Nitration Product, 3-Nitro-4-Acetamidophenol, Obtained From Acetaminophen With Nitrous AcidDocument2 pagesA New Nitration Product, 3-Nitro-4-Acetamidophenol, Obtained From Acetaminophen With Nitrous AcidxcvNo ratings yet
- Synthesis, Characterization and Thermal Behaviour PDFDocument6 pagesSynthesis, Characterization and Thermal Behaviour PDFxcvNo ratings yet
- Synthesis of DIAZOTETRAZOLE - PrepChemcomDocument2 pagesSynthesis of DIAZOTETRAZOLE - PrepChemcomxcvNo ratings yet
- Schweitzer C.E. Ethyleneurea. II.Document5 pagesSchweitzer C.E. Ethyleneurea. II.xcvNo ratings yet
- US2430874Document2 pagesUS2430874xcvNo ratings yet
- Synthesis, Characterization and Thermal BehaviourDocument6 pagesSynthesis, Characterization and Thermal BehaviourxcvNo ratings yet
- Heng Jiang Et Al. Tin Chloride Catalysed Oxidation of Acetone With Hydrogen Peroxide To Tetrameric Acetone PeroxideDocument2 pagesHeng Jiang Et Al. Tin Chloride Catalysed Oxidation of Acetone With Hydrogen Peroxide To Tetrameric Acetone PeroxidexcvNo ratings yet
- Azole (Wiki)Document2 pagesAzole (Wiki)xcvNo ratings yet
- US4094879Document4 pagesUS4094879xcvNo ratings yet
- Synthesis of Tetrazene - PrepChemcomDocument2 pagesSynthesis of Tetrazene - PrepChemcomxcvNo ratings yet
- Increasing the Tensile Strength and Lowering the Glass Transition Temperature of Composite Rocket PropellantsDocument34 pagesIncreasing the Tensile Strength and Lowering the Glass Transition Temperature of Composite Rocket PropellantsxcvNo ratings yet
- Synthesis of 5-Aminotetrazole PrepDocument3 pagesSynthesis of 5-Aminotetrazole PrepxcvNo ratings yet
- Potassium Azide (Wiki)Document1 pagePotassium Azide (Wiki)xcvNo ratings yet
- Peroxymonosulfuric Acid (Wiki)Document1 pagePeroxymonosulfuric Acid (Wiki)xcvNo ratings yet
- 1825 4875 1 SMDocument9 pages1825 4875 1 SMxcvNo ratings yet
- Formic Acid (Wiki)Document5 pagesFormic Acid (Wiki)xcvNo ratings yet
- Oxalic acid cleaning agentDocument5 pagesOxalic acid cleaning agentxcvNo ratings yet
- Green Oxidation of Alcohols by Using Hydrogen Peroxide in WaterDocument9 pagesGreen Oxidation of Alcohols by Using Hydrogen Peroxide in WaterxcvNo ratings yet
- Calcium nitrate: fertilizer, waste treatment, concrete hardenerDocument2 pagesCalcium nitrate: fertilizer, waste treatment, concrete hardenerxcvNo ratings yet
- Oxidation of Alcohols With Hydrogen Peroxide in The Presence of A New Triple-Site PhosphotungstateDocument11 pagesOxidation of Alcohols With Hydrogen Peroxide in The Presence of A New Triple-Site PhosphotungstatexcvNo ratings yet
- Peroxides PDFDocument16 pagesPeroxides PDFxcvNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (894)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- A Processing Guide For Injection Molding (Bayer)Document60 pagesA Processing Guide For Injection Molding (Bayer)Fabian Moffa100% (1)
- Carbonyl ChemistryDocument53 pagesCarbonyl Chemistrysujithhr1233395No ratings yet
- Product ListDocument48 pagesProduct ListRadisun LifesciencesNo ratings yet
- Chemistry WS 1-X, Chemical Reactions Equations-2019-20Document3 pagesChemistry WS 1-X, Chemical Reactions Equations-2019-20GsgshsjNo ratings yet
- Solutions, Chemical Reagents and Laboratory SuppliesDocument22 pagesSolutions, Chemical Reagents and Laboratory SuppliesStephen Janseen DelaPeña BaloNo ratings yet
- WC 500142231Document18 pagesWC 500142231abiazizNo ratings yet
- Aldehydes Ketones and Carboxylic Acids - NCERT SolutionsDocument27 pagesAldehydes Ketones and Carboxylic Acids - NCERT SolutionsVyjayanthiNo ratings yet
- TCA Cycle Notes1Document17 pagesTCA Cycle Notes1Krizzi Dizon GarciaNo ratings yet
- Sponsored by Ccps Supporters: Messages For Manufacturing PersonnelDocument1 pageSponsored by Ccps Supporters: Messages For Manufacturing PersonnelTriod jacksonNo ratings yet
- Exam 2 ChemistryDocument7 pagesExam 2 ChemistryEvelynNo ratings yet
- A Textbook Organic Chemistry - CompressedDocument1,224 pagesA Textbook Organic Chemistry - CompressedEJ CUBERNo ratings yet
- Mechanical Seals Reliability TQ-PJNDocument6 pagesMechanical Seals Reliability TQ-PJNPete PompesNo ratings yet
- Bomba de Pulmon PACA PACADocument24 pagesBomba de Pulmon PACA PACATaller cncNo ratings yet
- Carbohydrates Amino Acids Polymers IIT TheoryDocument53 pagesCarbohydrates Amino Acids Polymers IIT TheoryAshok PradhanNo ratings yet
- calibration of congo red colorDocument12 pagescalibration of congo red colorJelena MitrovicNo ratings yet
- Amoniaco SCCDocument96 pagesAmoniaco SCCmarcela celis100% (1)
- Guide To Laboratory Establishment For Plant Nutrient Analysis (PDFDrive)Document220 pagesGuide To Laboratory Establishment For Plant Nutrient Analysis (PDFDrive)amir_mNo ratings yet
- IBB MurialdoetalDocument25 pagesIBB MurialdoetalSil MuriNo ratings yet
- Neutralizing Amines: Boiler Water Operator Training NotesDocument4 pagesNeutralizing Amines: Boiler Water Operator Training NotesSheikh SahabNo ratings yet
- J.C. Bose University of Science and Technology, Ymca, FaridabadDocument18 pagesJ.C. Bose University of Science and Technology, Ymca, FaridabadSumit MeghNo ratings yet
- Inorganic Chemistry by Team Neet SecretDocument152 pagesInorganic Chemistry by Team Neet Secret09 Krishna TrivediNo ratings yet
- Effects of Neutralization and Bleaching Process On Fatty Acid and Triglyceride Compositions of Pomace-Olive OilDocument15 pagesEffects of Neutralization and Bleaching Process On Fatty Acid and Triglyceride Compositions of Pomace-Olive OilAhmed KhaledNo ratings yet
- Rubber Industries: A Concise History and OverviewDocument18 pagesRubber Industries: A Concise History and Overviewdaabgchi100% (1)
- Electrochemical Water Electrolysis-Fundamentals and Technologies-CRC PressDocument255 pagesElectrochemical Water Electrolysis-Fundamentals and Technologies-CRC PressRacle Gui100% (1)
- Introduction To Polymer ProcessingDocument43 pagesIntroduction To Polymer ProcessingShushil KumarNo ratings yet
- WaterDocument40 pagesWaterhimanshuchawla654No ratings yet
- Color Reactions R&DDocument2 pagesColor Reactions R&DJennifer HerediaNo ratings yet
- US5198281 Towpreg FabricDocument20 pagesUS5198281 Towpreg FabricyigitilgazNo ratings yet
- 3.17 Enthalpies of Solution: Using Hess's Law To Determine Enthalpy Changes of Solution Enthalpy of SolutionDocument4 pages3.17 Enthalpies of Solution: Using Hess's Law To Determine Enthalpy Changes of Solution Enthalpy of SolutionshowhardoNo ratings yet