0% found this document useful (0 votes)
829 views11 pagesHistopathology and Tissue Fixation Guide
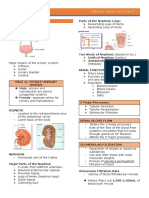
This document discusses histopathology and the process of tissue fixation. It describes how histotechnologists prepare tissue specimens for microscopic examination by pathologists. The key steps in tissue processing include gross examination, fixation, dehydration, clearing, and infiltration. Fixation is the first and most critical step, as it preserves the tissue morphology and prevents autolysis. The document outlines various types of fixatives, factors that influence fixation, and characteristics of good fixatives. The primary goal of fixation is to preserve tissues and prevent bacterial decomposition.
Uploaded by
army rebelCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
829 views11 pagesHistopathology and Tissue Fixation Guide
This document discusses histopathology and the process of tissue fixation. It describes how histotechnologists prepare tissue specimens for microscopic examination by pathologists. The key steps in tissue processing include gross examination, fixation, dehydration, clearing, and infiltration. Fixation is the first and most critical step, as it preserves the tissue morphology and prevents autolysis. The document outlines various types of fixatives, factors that influence fixation, and characteristics of good fixatives. The primary goal of fixation is to preserve tissues and prevent bacterial decomposition.
Uploaded by
army rebelCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd