Professional Documents
Culture Documents
Folic Acid Supplementation Recommendations2 by Indication
Folic Acid Supplementation Recommendations2 by Indication
Uploaded by
DrFarah Emad Ali0 ratings0% found this document useful (0 votes)
7 views1 pageObest
Original Title
Folic-acid-supplementation-recommendations2-by-indication
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentObest
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
7 views1 pageFolic Acid Supplementation Recommendations2 by Indication
Folic Acid Supplementation Recommendations2 by Indication
Uploaded by
DrFarah Emad AliObest
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
Folic acid supplementation recommendations by indication
Indication for supplementation Dose Start (minimum) Duration* Recommended
(daily) by
High risk
Prior open NTD in offspring of either parent or personal
history of open NTD in either parent
4 mg Three months PTC 12 weeks SOGC , ACOG
Women on valproate or carbamazepine
Type I or II diabetes 5 mg Three months PTC 12 weeks RCOG
Body Mass Index > 30 kg/m2 5 mg Three months PTC 12 weeks RCOG
Moderate risk
Personal or family history of folate-sensitive congenital 1 mg Three months PTC 12 weeks SOGC
anomaly other than NT
Family history of NTD ( first- or second- degree relative) 1 mg Three months PTC 12 weeks SOGC
Type I or II diabetes 1 mg Three months PTC 12 weeks SOGC
Women on other antiepileptic drugs 0.4 mg One month PTC 12 weeks ADA, ACOG
Maternal gastrointestinal malabsorption 1 mg Three months PTC 12 weeks SOGC
Medical conditions associated with risk (advanced liver 1 mg Three months PTC 12 weeks SOGC
disease, dialysis, alcohol overuse)
Low risk
Pregnancy or potential for pregnancy 0.4 mg At least one month PTC 12 weeks ACOG, CDC
0.4 - 0.8 One month PTC First 2-3 months of pregnancy USPSTF
mg
NTD: neural tube defect; PTC: prior to conception; ACOG: American College of Obstetricians & Gynecologists; SOGC: Society of Obstetricians & Gynecologists
of Canada; ADA: American Diabetes Association; RCOG: Royal College of Obstetricians & Gynecologists; USPSTF: United State Preventive Services Task Force;
CDC: Centers for Disease Control & Prevention. * After 12 weeks to continue on 0.4 mg with other supplement and as required.
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5807)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1091)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (842)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (346)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Renal Function TestsDocument19 pagesRenal Function TestsDrFarah Emad Ali100% (1)
- Increased COVID-19 Infections in Women With Polycystic Ovary Syndrome: A Population-Based StudyDocument9 pagesIncreased COVID-19 Infections in Women With Polycystic Ovary Syndrome: A Population-Based StudyDrFarah Emad AliNo ratings yet
- Metabolism Open: Abu Saleh MD Moin, Thozhukat Sathyapalan, Stephen L. Atkin, Alexandra E. ButlerDocument4 pagesMetabolism Open: Abu Saleh MD Moin, Thozhukat Sathyapalan, Stephen L. Atkin, Alexandra E. ButlerDrFarah Emad AliNo ratings yet
- Hepatic Circulation and Toxicology: Drug Metabolism Reviews February 1997Document28 pagesHepatic Circulation and Toxicology: Drug Metabolism Reviews February 1997DrFarah Emad AliNo ratings yet
- Ovulation Test: 5 Important Points You Need To ReadDocument2 pagesOvulation Test: 5 Important Points You Need To ReadDrFarah Emad AliNo ratings yet
- Technologies For Cervical Cancer Detection, Diagnosis, Monitoring and Treatment in Low-Resource SettingsDocument42 pagesTechnologies For Cervical Cancer Detection, Diagnosis, Monitoring and Treatment in Low-Resource SettingsDrFarah Emad AliNo ratings yet
- Pancreas: Anatomy, Diseases and Health Implications: September 2012Document53 pagesPancreas: Anatomy, Diseases and Health Implications: September 2012DrFarah Emad AliNo ratings yet
- Doses of Misoprostol Use Updated March 2018 PDFDocument2 pagesDoses of Misoprostol Use Updated March 2018 PDFDrFarah Emad AliNo ratings yet
- Multiple Choice Questions: Section 1: Androgen Production and MechanismsDocument4 pagesMultiple Choice Questions: Section 1: Androgen Production and MechanismsDrFarah Emad AliNo ratings yet
- Cognitive Assessment Tip SheetDocument1 pageCognitive Assessment Tip SheetDrFarah Emad AliNo ratings yet
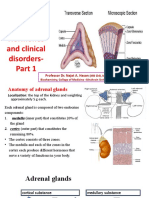
- Adrenal Gland: Structure, Hormones and Clinical Disorders-: Professor Dr. Najat A. HasanDocument18 pagesAdrenal Gland: Structure, Hormones and Clinical Disorders-: Professor Dr. Najat A. HasanDrFarah Emad Ali100% (1)
- Cardiac Function Tests: A Body and Disease WorkshopDocument32 pagesCardiac Function Tests: A Body and Disease WorkshopDrFarah Emad AliNo ratings yet
- Thyroid Gland and Its Rule in Human BodyDocument9 pagesThyroid Gland and Its Rule in Human BodyDrFarah Emad AliNo ratings yet
- Risk Assessment For Venous ThromboembolismDocument1 pageRisk Assessment For Venous ThromboembolismDrFarah Emad AliNo ratings yet
- Chapter-25 Kidney Function Tests: January 2017Document14 pagesChapter-25 Kidney Function Tests: January 2017DrFarah Emad AliNo ratings yet
- Ultrasound in Infertility PDFDocument11 pagesUltrasound in Infertility PDFDrFarah Emad AliNo ratings yet
- Depression and Multiple Sclerosis PDFDocument9 pagesDepression and Multiple Sclerosis PDFDrFarah Emad AliNo ratings yet
- Faten Imad Ali: Drug-Resistant Tuberculosis: How To Interpret Rapid Molecular Test ResultsDocument1 pageFaten Imad Ali: Drug-Resistant Tuberculosis: How To Interpret Rapid Molecular Test ResultsDrFarah Emad AliNo ratings yet
- COVID-19 Infection & Woman HealthDocument17 pagesCOVID-19 Infection & Woman HealthDrFarah Emad AliNo ratings yet
- Ofirmev Monograph FinalDocument40 pagesOfirmev Monograph FinalDrFarah Emad AliNo ratings yet
- Mobilis: An Osmoprotective Periplasmic Enzyme ContainingDocument16 pagesMobilis: An Osmoprotective Periplasmic Enzyme ContainingDrFarah Emad AliNo ratings yet