Professional Documents
Culture Documents
Jurnal 2 Cpo Deya
Jurnal 2 Cpo Deya
Uploaded by
MegaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Jurnal 2 Cpo Deya
Jurnal 2 Cpo Deya
Uploaded by
MegaCopyright:
Available Formats
730
G. SAROJINI, K. CHITTEMMA RAO, P.G. TULPULE AND G. LAKSHMINARAYANA
consumption. Hence, CPFA could not be a problem as 37:440 (1960).
these are eliminated in the cooking process. But epoxy oleic 4. Eder, S.R, and G. Guhr, Fette. Seifen Anstrichm. 81: 566
(1979).
acid could not be eliminated with any of the processing 5. Hopkins, c.Y., and M.J. Chisholm, JAOCS 36:95 (1959).
techniques. It is present in small quantities only, and its 6. Paal, C., and K. Roth, Chern. Abst. 3:2158 (1909).
level can be reduced further by blending with common 7. Official and Tentative Methods of the American Oil Chemists'
cooking oils. Processing of H. sabdariffa seed oil thus may Society (1973). Third edition. Vol. I and II. AOCS, Chicago.
Methods Ca 5a-40, Ca 61-52, Cc 7-25, Cc 109-25, Cc 9a-48,
render it suitable for human consumption. Cd 1-25, Cd 3-25, Cd 8-53.
8. Christie, W.W. (1973). Lipid analysis, isolation, separation,
identification and structural analysis of lipids. Pergamon Press,
ACKNOWLEDGMENTS Oxford, p. 90.
9. Schneider, E.L., S.P. Loke and D.T. Hopkins, JAOCS 45:585
The Andhra Pradesh Agricultural University provided the oppor-
(1968).
tunity to do the research and LC.A.R provided financial assistance.
Part of the work was done in the Regional Research Laboratory and 10. Fioriti, J.A., and RJ. Sims, J. Chromatog. 32:761 (1968).
National Institute of Nutrition. 11. Chakrabarty, M.M., D. Bhattacharya and M.K. Kundu, Analyst.
95:85 (1970).
12. Rukmini, C.M., Vijayaraghavan and P.G. Tulpule, JAOCS 59:
415 (1982).
REFERENCES 13. Sultana, S.N., and D.P. Sen, J. Fd. Sci. Tech. 16:208 (1979).
14. Vidyasagar, K., 5.5. Arya, K.S. Premavalli, D.B. Parihar and
1. Ramakrishna, G., N. Krishna Reddy, G. Azeemoddin, D. H. Nath, J. Fd. Sci. Tech. 11:73 (1974).
Atchyutaramayya and S.D. Thirumala Rao, J. Oil Tech. Assn. 15. Moore, H.K., G.A. Richter and W.B. Van Arsdel, Ind. Eng.
India. 11:75 (1979). Chern. 9:451 (1971).
2. Ahmad, M.U., S.K. Hussain, 1. Ahmad and S.M. Osman, J. Sci.
Fd. Agric. 30:424 (1979).
3. Earle, F.R., C.A. Glass, G.C. Gisinger and LA. Wolff, JAOCS [Received September 6,1983]
~Determinationof Mono- and Diglycerides in Palm Oil, Olein
and Stearin
E.M. GOH and R.E. TIMMS, Kempas Edible Oil Sdn. Berhad, P.O. Box 75, Pasir Gudang, Johore,
Malaysia.
ABSTRACT naturally contains this level of monoglycerides (5). Al-
though refining reduces the content of monoglycerides, the
Partial glycerides are important constituents of palm oil and can
have significant effects on the physical properties of products con- surface active properties of tnonoglycerides also are im-
taining palm oil or on the fractionation of palm oil. A method.is portant in the detergent fractionation of crude palm oil to
described for their routine determination in palm oil. By analysis of yield palm olein and stearin.
28 weekly composite samples of crude palm oil the following results In this paper we report the use of a gas liquid chroma-
were obtained: free fatty acids, mean = 3.76%, range 2.4 to 4.5%; tographic (GLC) method for the study of partial glycerides
monoglycerides, mean = 0.28%, range 0.21 to 0.34%; diglycerides, in crude, fractionated and refined palm oils. Trimethylsilyl
mean = 6.30%, range 5.3 to 7.7%. During detergent fractionation of (TMS) derivatives are prepared prior to GLC analysis.
palm oil, diglycerides concentrate in the palm olein, but monogly- Similar analytical methods using various silylating and GLC
cerides concentrate in the palm stearin. Palm fatty acid distillate was procedures have been reported previously (7,8,9,10,11).
found to contain approximately 3% each of mono- and diglycerides.
Because the refining and fractionation processes are continuous in
the refinery, it is not possible to follow a single identifiable batch of EXPERIMENTAL
crude palm oil through the refinery. To circumvent this problem,
crude palm oil, stearin and olein from the refinery' were bleached
Samples
and steam refined in the laboratory and the partial glyceride con- All samples of palm oil, palm olein and palm stearin were
tents determined at each stage of processing. Except for fractiona- taken from the storage tanks or directly from the refinery
tion, the content of glycerides did not change during processing. For
of Kempas Edible Oil Sdn. Bhd., Pasir Gudang, Johore,
oil, olein and stearin, monoglycerides were reduced significantly
both after bleaching and after steam refining. Malaysia.
Fractionation was by detergent fractionation (Alia-
Laval). Refining was by physical refining comprising de-
INTRODUCTION gumming (0.04-0.07% phosphoric acid at 85 C), bleaching
Partial glycerides are important constituents of oils, espe- (1-2% earth at 110 C), and steam refining/deodorization at
cially palm oil, and they can have significant effects on 270 C (EMI).
physical properties. The lifetimes of Q polymorphs in palm
oil (1, 2, 3) and in shea fat (4) were influenced significantly Analysis of Monoglycerides, Diglycerides and FFA
by the level of diglycerides in the oils. Palm oil is unusually Preparation of TMS derivatives. 30 mg of the oil are
rich in partial glycerides (5), and their level is probably weighed accurately (±0.1 mg) into a 2 ml screw-capped
commercially important since partial glycerides also affect glass vial fitted with a septum (Supelco, Inc., Bellefonte,
the solid fat contents at all temperatures. Hernqvist and Pennsylvania, Catalog No. 3-3113). 400 J.1L of pyridine are
Anjou (6) have used diglycerides successfully to stabilize added using a syringe and the vial capped and shaken until
the {j' polymorph in margarines containing hydrogenated the oil dissolves. 100 J.1L· of N-trimethylsilyl imidazole
rapeseed and soyabean oils. In a margarine stored at 20 C, (Sigma Chemical Co., Saint Louis, Missouri) are then added
development of the {j polymorph could be delayed from through the septum using a syringe and needle. The vial is
four to 44 weeks by the addition of 5% diglycerides. then shaken well for one min. Finally, 200 J.1L of the inter-
Monoglycerides are u8ed at low levels, typically 0.3%, nal standard solution [250.0 mg n-triacontane (Sigma
to stabilize the oil/water emulsion in margarine. Palm oil Chemical Co.) in 25 ml of iso-octane] are added, again
JAOCS, Vol. 62, no. 4 (April 1985l
731
MONO- AND DIGLYCERIDES IN PALM OIL
TABLE I
Retention Times and Observed and Theoretical Response Factors
Retention Relative Response Factor
Time Retention
(seconds) Time Theoretical Observed
Triacontane 700 1 1 1
Palmitic Acid 188 0.27 1.011
Oleic Acid 276 0.39 1.002 } 1.084
Monopalmitin 504 0.72 0.977 1.003
Monoolein 589 0.84 0.973 0.998
Dipalmitin 1091 1.56 1.121 1.231
Diolein 1214 1.73 1.102 1.209
through the septum. Solutions were used within four hrs. deviation) to be: diglycerides ±0.31, monoglycerides ±0.03,
free fatty acids ±0.30.
Gas liquid chromatography. The TMS derivatives were
analyzed on a Hewlett-Packard Model 5790 gas chromato-
Laboratory Refining
graph. Column: glass, 0.5 m X 4 mm id, packed with 3%
OV-1 on Gas Chrom Q (Supelco). Chromatography condi- 500 g of crude oil were heated to 70 C and mixed with
tions: detector (FlO) and injector at 360 C, oven pro- 0.04% of 85% phosphoric acid for 15-20 min. The oil was
grammed from 150 C to 355 Cat 8 C/min followed by 6.5 then bleached with 2% Fulmont AA (Laporte), earth at
min at 355 C, carrier gas nitrogen at 60 mllmin. Peak areas 110 C for 30 min. After filtering, the bleached oil was
and retention times were determined using a Hewlett- steam refined under reduced pressure (2-5 torr) at 265 C
Packard 3390A integrator. For GLC analysis, a 2 JJ.L sam- for one hr.
ple of the solution was injected into the gas chromatograph.
Standard mono- and diglycerides (Nu-Chek Prep. Inc., RESULTS AND DISCUSSION
Elysian, Minnesota) were used to obtain the relative reten-
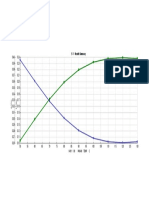
tion times and response factors required to calibrate the A typical chromatogram for the analysis of crude palm oil
method. is shown in Figure 1. Total analysis time is less than 45 min
including sample preparation, GLC analysis and cooling of
Calculation of results. Theoretical response factors for the GLC oven ready for the next analysis. An analysis of
triacontane, monoglycerides,' diglycerides and free fatty triglycerides by carbon number also is obtained, although in
acids were calculated using the equation: our laboratory we prefer to obtain this analysis indepen-
dently by tern perature programming from 295 to 355 C
Molecular Weight TG
Response factor = W .........
where W = weight of all carbon atoms in the TMS deriva-
tive, excluding the carbonyl carbons.
Actual response factors were obtained using known
amounts of the standard partial glycerides which were
silylated using the same procedure as for an oil sample. For
free fatty acids, a response factor was obtained by com-
parison of the GLC result with the FFA determined by the
standard titration procedure (AOCS Official Method Ca
IS
5a-40). Free fatty acids were calculated as palmitic acid.
Retention times and response factors are given in Table
I. Observed response factors were up to 10% higher than w
VI
theoretical response factors, but the relative magnitudes for Z
0
acids, mono- and diglycerides were as predicted. For prac- 0..
VI
tical purposes it was considered sufficient to use a single W
Q:
response factor for each lipid class, namely: monogly-
cerides, 1.00; diglycerides, 1.22, and free fatty acids, 1.08. ci FFA
,....L..,
These factors were then used in the following equation to
give the percentage by weight of each component: u.:
A(G) X f X W(TC) X 100
%= A(TC) X W(S)
where A(G) = area of glyceride (or FFA) peak from inte-
grator; A(TC) = area of triacontane peak from integrator;
W(TC) = weight of triacontane added = 2 mg in above pro- o 20 30
cedure; W(S) = weight of oil sample taken = 30 mg in above TIME (MINS)
procedure, and f = response factor as above.
By 10 replicate analyses of a sample of crude palm FIG. 1. Chromatogram of crude palm oil containing 0.3% monogly-
stearin, analytical errors for single determinations were esti- cerides (MG), 5.9% diglycerides (DG) and 3.5% free fatty acids
mated as 95% confidence limits (t at P=0.05 X standard (FFA). TG =ttiglycerides and IS =internal standard.
JAOCS, Vol. 62, no. 4 (April 1985l
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5820)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (845)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (540)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (348)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (822)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Soil Science MCQs With Answers-1Document143 pagesSoil Science MCQs With Answers-1Amokhan Amolkhan90% (21)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Problem Set On Redox TitrationsDocument4 pagesProblem Set On Redox Titrationsjaksj kkkksNo ratings yet
- MSDS Gamma TocotrienolDocument6 pagesMSDS Gamma TocotrienolMegaNo ratings yet
- Nama: Farah Alliya Yusda (05161024) Yulia Anngraini (05161080)Document1 pageNama: Farah Alliya Yusda (05161024) Yulia Anngraini (05161080)MegaNo ratings yet
- Table of Laplace Transforms PDFDocument1 pageTable of Laplace Transforms PDFMegaNo ratings yet
- Petroleum Refinning: Don't Be Afraid of Failure, This Is The Way To SucceedDocument12 pagesPetroleum Refinning: Don't Be Afraid of Failure, This Is The Way To SucceedMegaNo ratings yet
- Batch Distillation - WikipediaDocument9 pagesBatch Distillation - Wikipediaprince christopherNo ratings yet
- Alkaline Depolymerization of Polyethylene Terephthalate Plastic WasteDocument9 pagesAlkaline Depolymerization of Polyethylene Terephthalate Plastic WasteyuppeNo ratings yet
- SOP Testing Water Wastewater Treatment ChemicalsDocument56 pagesSOP Testing Water Wastewater Treatment ChemicalslaurianNo ratings yet
- Electrochemistry - TutorialDocument3 pagesElectrochemistry - TutorialHarsha DananjayaNo ratings yet
- Feasibility Study of ..Document108 pagesFeasibility Study of ..mohanadNo ratings yet
- ReactivesiliconesDocument59 pagesReactivesiliconesVaishali PuriNo ratings yet
- Astm D6474-20Document3 pagesAstm D6474-20wenhsiaochuanNo ratings yet
- Use of Industrial Waste - (Red Mud) in The Production of Self Compacting ConcreteDocument8 pagesUse of Industrial Waste - (Red Mud) in The Production of Self Compacting ConcreteIJRASETPublicationsNo ratings yet
- BS en 13137-2001Document24 pagesBS en 13137-2001ASESORIAS SOLDADURAS100% (1)
- Electrode ManufacturerDocument6 pagesElectrode ManufacturerHARSH PUNMIYANo ratings yet
- Heat Energy NotesDocument50 pagesHeat Energy Notesopoku jonathanNo ratings yet
- Nomenclature of Organic CompoundsDocument34 pagesNomenclature of Organic Compoundstapas kunduNo ratings yet
- Liquid-Liquid Equilibria For Ternary System of DME+METHANOL+WATER AT 313KDocument5 pagesLiquid-Liquid Equilibria For Ternary System of DME+METHANOL+WATER AT 313Ksweety vermaNo ratings yet
- F13005001-C001 MOLI (Form B) DIN 42569 RadialDocument1 pageF13005001-C001 MOLI (Form B) DIN 42569 RadialAngel StragliatiNo ratings yet
- Faculty of ScienceDocument44 pagesFaculty of ScienceSALAH EDDINENo ratings yet
- Experimental Optimization of The Disinfection Performance of Sodium Hypochlorite and Hypochlorous Acid in Pilot and Industrial Cooling TowersDocument11 pagesExperimental Optimization of The Disinfection Performance of Sodium Hypochlorite and Hypochlorous Acid in Pilot and Industrial Cooling Towersjuli sanchezNo ratings yet
- ASTM C233-18 Standard Test Method For Air-Entraining Admixtures For ConcreteDocument6 pagesASTM C233-18 Standard Test Method For Air-Entraining Admixtures For Concretebenedick barquinNo ratings yet
- Biochemistry Lab Manual of Post RN of KGH SONDocument71 pagesBiochemistry Lab Manual of Post RN of KGH SONKhurram Paul100% (1)
- Inorganic Chemistry MP 1Document6 pagesInorganic Chemistry MP 1ahmedali51367No ratings yet
- Polyphony: Superposition Independent Methods For Ensemble-Based Drug DiscoveryDocument19 pagesPolyphony: Superposition Independent Methods For Ensemble-Based Drug DiscoveryIarinaNo ratings yet
- PT 1Document26 pagesPT 1Roronoa ZoroNo ratings yet
- Awazel AWATEX RBE 6500 Rubberized Damp Proof Waterproof Bituminous EmulsionDocument2 pagesAwazel AWATEX RBE 6500 Rubberized Damp Proof Waterproof Bituminous Emulsionabdo emadNo ratings yet
- Narayana Junior College: Narayanaguda Division Senior Inter: Chemistry Ipe Important QuestionsDocument4 pagesNarayana Junior College: Narayanaguda Division Senior Inter: Chemistry Ipe Important Questionskeerth50% (2)
- Certificate of Accreditation: Perry Johnson Laboratory Accreditation, IncDocument26 pagesCertificate of Accreditation: Perry Johnson Laboratory Accreditation, IncVictor Arciga GranadosNo ratings yet
- Skala MohsDocument2 pagesSkala Mohssandy widodoNo ratings yet
- ACI 517-2R-87 Standard Specification For Tolerances For Accelerated Curing at Atmospheric PressureDocument17 pagesACI 517-2R-87 Standard Specification For Tolerances For Accelerated Curing at Atmospheric PressureKiramat ShahNo ratings yet
- Analyzing The Statistical Error of Physical Chemistry Experimental DataDocument7 pagesAnalyzing The Statistical Error of Physical Chemistry Experimental DataNurin BatrisyiaNo ratings yet
- Bachelor of Pharmacy: Aks University, SatnaDocument93 pagesBachelor of Pharmacy: Aks University, SatnaPrathiNo ratings yet