Professional Documents
Culture Documents
Assignment 1 PDF
Uploaded by
Sandeep ChallaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Assignment 1 PDF
Uploaded by
Sandeep ChallaCopyright:
Available Formats
CLL727 Assignment #1 Submission Due date January 29, 2019
1. Using any computational method evaluate the Thermodynamic equilibrium composition for the
steam reforming of methanol, assuming contribution of water gas shift reaction, and CO2 hydrogenation
reaction for methane formation. Plot conversion and selectivity of hydrogen as a function of
temperature.
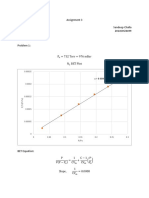
2. The adsorption of CO on activated carbon was followed experimentally at 0ºC. At the given pressures,
the following quantities of adsorbed gas were measured (corrected to a standard pressure of 1 bar):
P mbar 133 267 400 533 667 800 933
V cm3 10.3 19.2 27.3 34.0 40.1 45 49
Determine whether the measurements conform to the Langmuir isotherm and calculate
a) the constant KA and
b) the volume corresponding to complete coverage.
3. In carrying out heterogeneously catalyzed reactions, a distinction is made between microkinetics and
macrokinetics. Which steps have to be taken into account in the case of microkinetics? How Density
functional calculations are helpful in identifying a catalyst material for the reaction.
4. A methanation reaction was investigated on a commercial supported catalyst 0.5 % Rh/Al2O3:
CO + 3 H2 -------- CH4 + H2O
The degree of dispersion D of the the catalyst was found to be 42 % by means of chemisorption
measurements with H2. At 10 bar and 300ºC a catalyst turnover number of 0.16 s-1 was determined for
methane. Calculate the rate of formation of methane rCH4 in mol s-1g(cat.)-1 (metal + support).
5. Explain the principle of Sabatier for relating turn over frequency with binding energy over different
metals. What is compensation effect.
You might also like
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- BJHDocument8 pagesBJHElisabeth Kurnia BloomNo ratings yet
- Cover LetterDocument1 pageCover LetterSandeep ChallaNo ratings yet
- Assignment 3Document4 pagesAssignment 3Sandeep ChallaNo ratings yet
- NumbersDocument1 pageNumbersSandeep ChallaNo ratings yet
- Handouts PDFDocument53 pagesHandouts PDFSandeep ChallaNo ratings yet
- L26 27 PDFDocument35 pagesL26 27 PDFSandeep ChallaNo ratings yet
- 3 2PPDocument3 pages3 2PPSandeep ChallaNo ratings yet
- 3 2PPDocument3 pages3 2PPSandeep ChallaNo ratings yet
- Catalysis Arrhenius PDFDocument14 pagesCatalysis Arrhenius PDFSandeep ChallaNo ratings yet
- Interpretation:: Lewis Base Lewis AcidDocument4 pagesInterpretation:: Lewis Base Lewis AcidSandeep ChallaNo ratings yet
- Flame AA ExperimentDocument4 pagesFlame AA ExperimentSandeep ChallaNo ratings yet
- Interpretation:: CH NH BFDocument2 pagesInterpretation:: CH NH BFSandeep ChallaNo ratings yet
- Advance Reaction Engineering Analysis - Assignment 3Document5 pagesAdvance Reaction Engineering Analysis - Assignment 3Sandeep ChallaNo ratings yet
- Assignment 2 3Document3 pagesAssignment 2 3Sandeep Challa0% (1)
- Lean manufacturing waste identification using COMMWIPDocument3 pagesLean manufacturing waste identification using COMMWIPSandeep ChallaNo ratings yet
- 3rd Example-Assignment 2Document2 pages3rd Example-Assignment 2Sandeep ChallaNo ratings yet
- Chemical EngineeringDocument1 pageChemical EngineeringRahmatullah Jami'inNo ratings yet
- Sandeep Challa LORDocument2 pagesSandeep Challa LORSandeep ChallaNo ratings yet
- B.tech Chemical DatabaseDocument5 pagesB.tech Chemical DatabaseSandeep ChallaNo ratings yet
- Troubleshooting - Honeycomb and Voids - tcm45-589731Document1 pageTroubleshooting - Honeycomb and Voids - tcm45-589731Behdad_MNo ratings yet
- Chemcad 6 User GuideDocument202 pagesChemcad 6 User Guideerhan ünal100% (1)
- Distillation Column DesignDocument20 pagesDistillation Column DesignSandeep Challa100% (1)
- Role of Automation in Industries by RajeshDocument41 pagesRole of Automation in Industries by RajeshSandeep ChallaNo ratings yet
- DHDS unit process descriptionDocument9 pagesDHDS unit process descriptionSandeep ChallaNo ratings yet
- Effect of Interfacial Surface Tension On Flow Inside A Cavity Filled With Immiscible Liquids.Document7 pagesEffect of Interfacial Surface Tension On Flow Inside A Cavity Filled With Immiscible Liquids.Sandeep ChallaNo ratings yet
- Raw Materials ImpDocument6 pagesRaw Materials ImpSandeep ChallaNo ratings yet
- OrientDocument20 pagesOrientSandeep ChallaNo ratings yet
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)