Professional Documents
Culture Documents
LAB
Uploaded by
Wonda 005Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
LAB
Uploaded by
Wonda 005Copyright:
Available Formats
1. Start Up procedure.
I. Ensure all valves are closed.
II. Prepare:
a. Prepare 1 L of 0.1M of Sodium Hydroxide [NaOH]
b. Prepare 1 L of 0.1M of Ethyl Acetate [Et (Ac)]
c. Prepare 1 L of deionized water [H2O]
III. Fill the feed vessel B1 and B2 with NaOH solution [Et (Ac)] and respectively.
IV. Turn on the power of control panel.
V. Open V1, V2 and V27.
VI. Switch on pump 1 [P1] and pump 2 [P2].
VII. Make sure pipeline before V9 and V10 are filled with liquid. Any trap air should be
release using P1 and P2.
VIII. The flow of NaOH and [Et (Ac)] solution is being observed into the dosing vessel
[HB1 and HB2] through V10 and V9, respectively.
IX. Let both solution in dosing vessel [HB1 and HB2] to overflow into the feed vessel [B1
and B2 respectively].
Shut down procedure.
I. Switch of pump 1 [P1] and pump 2 [P2]
II. Closed valve on V27.
III. Drain all liquid from the unit by open V3 to V21 and V26.
IV. Turn off the power from the control panel.
Selecting suitable reactor.
I. Make sure V3 to V21 are close.
II. For plug flow reactor [SR1],
a. V11 and V19 are open.
b. Record the outlet conductivity value [QI-404] and temperature value [TI-104].
III. For single batch reactor [R1]
a. Ensure V13, V14 and V20 are opened.
b. Switch on the stirrer [M1]
c. Record the outlet conductivity value [QI-402] and temperature value [TI-102].
IV. For 3-stage continuous stirred tank reactor [R2, R3 and R4]
a. V12 and V21 are opened.
b. Switch on the stirrer [M2, M3 and M4].
c. Record the outlet conductivity value [QI-403] and temperature value [TI-103]
2. Plan to minimize the usage of raw material.
a. Reduce the amount of raw material used as long the concentration is the same.
b. Prepare the raw material as indicated in the startup procedure.
c. Avoid careless mistake [spill a small amount of the raw material].
3. Method on validating the accuracy of flow and conductivity meter of test reactor.
a. The conductivity of NaOH concentration from each conversion value were determined
by mixing the following solution into 100 mL of deionized water:
i. O% conversion = 100 mL NaOH
ii. 25% conversion = 75 mL NaOH + 25 mL [Et (Ac)]
iii. 50% conversion = 50 mL NaOH + 50 mL [Et (Ac)]
iv. 75% conversion = 25 mL NaOH + 75 mL [Et (Ac)]
v. 100% conversion = 100 mL [Et (Ac)]
4. Experimental methodology to identify the overall reaction order and the specific reaction
rate for the saponification reaction between NaOH and [Et (Ac)]
5. Experimental methodology to determine the type of reactor which most suitable for
saponification reaction between NaOH and [Et (Ac)].
a. Perform the general start-up procedure.
b. Select the desire reactor as stated in the selecting the reactor.
c. Open valve V9 and V10.
d. Allow the NaOH and [Et (Ac)] solution to enter the selected reactor and make sure the
receiving vessel [B3] are empty.
e. Control the flowrate by adjusting valve V9 and V10. Record the flowrate and make sure
the flowrate is same.
f. To ensure the reactor has reach steady state, record the inlet and outlet conductivity
values of the selected reactor for every 5 minutes until they are at a steady state.
g. Repeat the experiment from b until f by selecting a different reactor.
6. Suggest the most suitable reactor for saponification of NaOH and [Et (Ac)].
Three continuous stirred tank reactors.
I. CSTR always operate at steady state.
II. Inside the reactor are assumed to be perfectly mixed.
III. Saponification process is a continuous reaction.
You might also like
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (589)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (842)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5806)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1091)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Sulphonation Ballestra PDFDocument20 pagesSulphonation Ballestra PDFmahmoud100% (2)
- Francis X. McConville - The Pilot Plant Real Book A Unique Handbook For The Chemical Process Industry (2002, FXM Engineering and Design) PDFDocument309 pagesFrancis X. McConville - The Pilot Plant Real Book A Unique Handbook For The Chemical Process Industry (2002, FXM Engineering and Design) PDFHaziq100% (3)
- Workbook For Chemical Reactor Relief System Sizing PDFDocument256 pagesWorkbook For Chemical Reactor Relief System Sizing PDFTran Van HaiNo ratings yet
- Kinetics Ans Key Master FileDocument10 pagesKinetics Ans Key Master FileJOANA RHEA SAGPAEYNo ratings yet
- HYSYS-Report Ammonia PlantDocument21 pagesHYSYS-Report Ammonia PlantDouglas Ross HannyNo ratings yet
- Jacketed VesselDocument4 pagesJacketed Vesselnithansa100% (1)
- Cost Reduction in Polysilicon Manufacturing For PhotovoltaicsDocument25 pagesCost Reduction in Polysilicon Manufacturing For PhotovoltaicsMohamed Farag MostafaNo ratings yet
- Review On Development of Polypropylene Manufacturing ProcessDocument11 pagesReview On Development of Polypropylene Manufacturing ProcessShweta Yadav100% (1)
- Credit To Beauty of Science, 2014Document12 pagesCredit To Beauty of Science, 2014Wonda 005No ratings yet
- NKB 30303 Environmental Issues and Waste Management: Sludge Treatment & DisposalDocument6 pagesNKB 30303 Environmental Issues and Waste Management: Sludge Treatment & DisposalWonda 005No ratings yet
- Aspen Tutorial #4: Design Specs & Sensitivity Analysis: OutlineDocument11 pagesAspen Tutorial #4: Design Specs & Sensitivity Analysis: OutlineWonda 005No ratings yet
- CnI Problem Statement 1Document1 pageCnI Problem Statement 1Wonda 005No ratings yet
- Aspen Tutorial 3Document10 pagesAspen Tutorial 3Wonda 005No ratings yet
- NKB 30303 Environmental Issues and Waste Management: Types of Solid WasteDocument11 pagesNKB 30303 Environmental Issues and Waste Management: Types of Solid WasteWonda 005No ratings yet
- NKB 30303 Environmental Issues and Waste Management: Measures of Water QualityDocument7 pagesNKB 30303 Environmental Issues and Waste Management: Measures of Water QualityWonda 005No ratings yet
- IP Law For EngineerDocument6 pagesIP Law For EngineerWonda 005No ratings yet
- NKB 30303 Environmental Issues and Waste Management: Major Air PollutantsDocument17 pagesNKB 30303 Environmental Issues and Waste Management: Major Air PollutantsWonda 005No ratings yet
- NCB40102 L5 Cyf OshDocument21 pagesNCB40102 L5 Cyf OshWonda 005No ratings yet
- IP Enforcement EIS2020Document21 pagesIP Enforcement EIS2020Wonda 005No ratings yet
- Design of Ideal Reactor For Single Reaction: Prepared By: Arbanah Muhammad Salmi Nur Ain SanusiDocument29 pagesDesign of Ideal Reactor For Single Reaction: Prepared By: Arbanah Muhammad Salmi Nur Ain SanusiWonda 005No ratings yet
- Chapter 4-: Determination of Rate Law For Batch ReactorDocument25 pagesChapter 4-: Determination of Rate Law For Batch ReactorWonda 005No ratings yet
- Solve: Assignment 2 (Laplace Transform & Differential Equations)Document1 pageSolve: Assignment 2 (Laplace Transform & Differential Equations)Wonda 005No ratings yet
- Stoichiometric Table & Material Balance: Prepared By: Arbanah MuhammadDocument24 pagesStoichiometric Table & Material Balance: Prepared By: Arbanah MuhammadWonda 005No ratings yet
- P - H Diagram R134a For Mechanical Heat PumpDocument2 pagesP - H Diagram R134a For Mechanical Heat PumpWonda 005No ratings yet
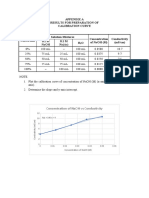
- Appendix A Results For Preparation of Calibration Curve: Concentration of Naoh Vs ConductivityDocument1 pageAppendix A Results For Preparation of Calibration Curve: Concentration of Naoh Vs ConductivityWonda 005No ratings yet
- ReferenceDocument1 pageReferenceWonda 005No ratings yet
- Tabulated Data: Amount of Caffeine in A ConcentrationDocument1 pageTabulated Data: Amount of Caffeine in A ConcentrationWonda 005No ratings yet
- Chapter Overview: 2: Modeling BasicsDocument84 pagesChapter Overview: 2: Modeling BasicsGigiNo ratings yet
- CSTR 40 LDocument28 pagesCSTR 40 LCik Tiem Ngagiman50% (2)
- Chem ScheduleDocument10 pagesChem ScheduleAnjali SindhuNo ratings yet
- Gate 1992 PDFDocument12 pagesGate 1992 PDFVammsy Manikanta SaiNo ratings yet
- CRE PPT - PPT NewDocument12 pagesCRE PPT - PPT NewmanavNo ratings yet
- Lecture 6 Catalytic Reforming ProcessDocument18 pagesLecture 6 Catalytic Reforming ProcessGAMERS OF KUWAITNo ratings yet
- A Study On Naphtha Catalytic Reforming Reactor Simulation and AnalysisDocument8 pagesA Study On Naphtha Catalytic Reforming Reactor Simulation and AnalysisAbdallah R. AwadNo ratings yet
- Us 5231222Document11 pagesUs 5231222Ratu TiaraNo ratings yet
- Chap 15 Marlin 2002Document49 pagesChap 15 Marlin 2002Audrey Patrick KallaNo ratings yet
- CPDS Tutorial 1Document32 pagesCPDS Tutorial 1MuhammadTanzeeLUsmanNo ratings yet
- Conceptual Modelling and Optimization of Jacketed Tubular Reactors For The Production of LDPEDocument6 pagesConceptual Modelling and Optimization of Jacketed Tubular Reactors For The Production of LDPECheak TingNo ratings yet
- Chemical Reaction PDFDocument4 pagesChemical Reaction PDFonyxNo ratings yet
- Week 4 - Isothermal Reactor Design (Part I)Document51 pagesWeek 4 - Isothermal Reactor Design (Part I)NadineNo ratings yet
- Reactor Design Guide1Document3 pagesReactor Design Guide1Muhammad NaqviNo ratings yet
- United States Patent: Ca (OH) 2, P7 CasoDocument8 pagesUnited States Patent: Ca (OH) 2, P7 CasoViolanda PranajayaNo ratings yet
- Bits PilaniDocument35 pagesBits PilaniPallavi ShaktawatNo ratings yet
- Catalytic Oxidation of Benzene To Maleic Anhydride in A Continuous Stirred Tank ReactorDocument7 pagesCatalytic Oxidation of Benzene To Maleic Anhydride in A Continuous Stirred Tank ReactorMirko GraneseNo ratings yet
- Ind. Eng. Chem. Res. 53 (2014) 18526 18538Document13 pagesInd. Eng. Chem. Res. 53 (2014) 18526 18538Shabih Ul HasanNo ratings yet
- WurzelDocument45 pagesWurzelCarmen Huaniquina TerrazasNo ratings yet
- A R K K: Chbe 6300: Kinetics and Reactor Design Homework 1Document2 pagesA R K K: Chbe 6300: Kinetics and Reactor Design Homework 1AnnNo ratings yet
- Styrene Methods 2520of ProductionDocument9 pagesStyrene Methods 2520of ProductionMohd Zulazreen50% (2)
- Simulation of A Urea Synthesis Reactor. Reactor Model: Horacio Irazoqui' and Miguel IslaDocument10 pagesSimulation of A Urea Synthesis Reactor. Reactor Model: Horacio Irazoqui' and Miguel IslaStephanie TraversNo ratings yet