Professional Documents
Culture Documents
Imaging in Acute Renal Infection: BJU International (2000), 86 Suppl. 1, 70 79
Imaging in Acute Renal Infection: BJU International (2000), 86 Suppl. 1, 70 79
Uploaded by
Gordana PuzovicOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Imaging in Acute Renal Infection: BJU International (2000), 86 Suppl. 1, 70 79
Imaging in Acute Renal Infection: BJU International (2000), 86 Suppl. 1, 70 79
Uploaded by
Gordana PuzovicCopyright:
Available Formats
BJU International (2000), 86 Suppl.
1, 70±79
Imaging in acute renal infection
A . K A W AS H I M A * { , C. M . SA N D L E R * { { and S . M . G O LD M A N* {
Departments of *Radiology and {Urology, The University of Texas-Houston Medical School, and {Department of Radiology,
Lyndon B. Johnson General Hospital, Houston, Texas, USA
acute focal bacterial nephritis and acute lobar nephronia
Introduction
[17,18]. In addition, with the advent of CT and
The urinary tract is one of the most common sites of ultrasonography (US), subtle abnormal nephrographic
infection in humans. Acute pyelonephritis in children ®ndings were present and variably termed cellulitis,
usually occurs because of UTI associated with VUR, while carbuncle, renal phlegmon, etc. [2,5]. To simplify the
in adults, frank re¯ux is rarely detected. Women of terminology, Talner et al. [2] recommended that all
< 50 years of age are more commonly affected by acute parenchymal abnormalities with no abscess attributable
pyelonephritis than men. When older than this, the to acute infection be called acute pyelonephritis and that
incidence of UTI in men increases as a result of urinary the extent or degree of involvement should be described
stasis secondary to prostatic hyperplasia and other by one or more of the following modi®ers; (i) unilateral or
factors [1,2]. bilateral, (ii) focal or diffuse, (iii) focal swelling or no
Acute pyelonephritis is a bacterial infection of the focal swelling, and (iv) renal enlargement or no focal
kidney that causes a tubulo-interstitial in¯ammation of enlargement. This classi®cation is used in the remainder
the renal parenchyma. Acute renal infection is of vary- of this article.
ing severity, from uncomplicated acute pyelonephritis
through progressively worsening stages of interstitial
Renal imaging in acute renal infection
in¯ammation to frank abscess formation [3,4]. Because
histological specimens are dif®cult to obtain, exact
Acute pyelonephritis in the adult
clinical correlation with these various stages of in¯am-
mation is impossible. Therefore, the primary goal of renal In most cases, UTI can be diagnosed in adults from the
imaging is to provide information about the nature and clinical and laboratory ®ndings, and imaging is usually
extent of the disease and to identify signi®cant complica- unnecessary. However, imaging is indicated when adult
tions, e.g. gas-forming infection, abscess and urinary patients with UTI respond poorly to appropriate antibiotic
obstruction [2,3,5±15]. therapy after 3 days [7,13,14,19]. In addition, when a
This article presents a review of the current role of and de®nite diagnosis of acute renal infection is not
controversies in imaging the kidneys to evaluate patients established or when patients present with recurrent
with acute renal infection. The nomenclature in describ- episodes of infection, renal imaging is indicated because
ing the extent of the renal imaging ®ndings in acute there is more likelihood of stones, obstruction, abscess, or
pyelonephritis suggested by Talner et al. is used, as a congenital anomaly. Among patients who have a
described below [2]. history of poorly controlled diabetes mellitus, AIDS, renal
transplantation, or other immunocompromised disease
states, there is an increased risk of developing a
Radiological terminology in acute renal
complicated UTI, including a renal or perinephric
infection
abscess. Therefore, imaging may be required when
Previously there has been much confusion about the such patients present initially [8±10,20,21].
terminology used to describe the extent and severity of CT is currently considered to be better than excretory
acute pyelonephritis. Classi®cations used by radiologists urography [22] and US [6,7,14] in detecting the
were based solely on the radiological features with no parenchymal abnormalities caused by renal infection
histological con®rmation. The advanced generalized form and in delineating the extent of the disease. Unenhanced
of acute pyelonephritis in diabetic patients, usually with a CT is useful in detecting calculi, gas-forming infections,
nonfunctioning kidney on excretory urography, was haemorrhage [23], parenchymal calci®cations, obstruc-
originally described as acute bacterial pyelonephritis tion and in¯ammatory masses. A contrast-enhanced
[16]. The localized form of acute pyelonephritis resulting study is essential to completely evaluate patients with
in a mass effect on imaging studies was described with a renal in¯ammatory disease, to de®ne changes in the
variety of terms, including acute focal pyelonephritis, renal excretion of the contrast material which occur as a
70 # 2000 BJU International
IMAGING IN ACUTE RENAL INFECTION 71
result of the in¯ammation. The most common CT
®ndings are ill-de®ned wedge-shaped lesions of decreased
attenuation radiating from the papilla in the medulla
to the cortical surface, with or without swelling.
Occasionally, linear bands of alternating hyper- and
hypo-attenuation orientated parallel to the axes of the
tubules and collecting ducts are seen. These CT ®ndings
have been attributed to diminished concentration of
contrast material in the tubules caused by tubular
obstruction with in¯ammatory cells and debris, ischae-
mia and interstitial oedema [2,24±26]. Small low-density
zones arising from tissue breakdown may resolve with
treatment or may coalesce to form a frank abscess.
With the introduction of the helical (or spiral) fast-
scanning technology, a particular phase of contrast
a
medium excretion can be imaged [27]. Renal vascularity
is evaluated during the vascular phase, 10±15 s after the
initiation of intravenous (IV) contrast-medium adminis-
tration. A dense cortical nephrogram with cortico-
medullary differentiation is obtained during the
corticomedullary differentiation nephrographic phase,
20±45 s after beginning the IV injection with contrast
medium. A homogeneous nephrogram can be obtained
during the generalized homogeneous nephrographic
phase 45±120 s after IV contrast medium administra-
tion. Contrast material starts to appear in the collecting
system in the excretory phase, >2 min after adminis-
tering contrast medium [8]. Routine helical CT of the
abdomen usually involves a monophasic examination
performed either during the corticomedullary differentia-
tion or generalized homogeneous nephrographic phase. b
Enhanced helical scans obtained during the corticome-
dullary differentiation and early generalized homoge-
neous nephrographic phase may show lobar or sublobar
areas of hypo-attenuating cortex with ill-de®ned corti-
comedullary differentiation [8±10]. Nephrographic
abnormalities of acute pyelonephritis are best seen on
CT scans obtained during the generalized homogeneous
nephrographic phase (Fig. 1) [28,29]. Excretory-phase
studies are less equivocal for diagnosing renal abscesses
than scans taken soon after contrast medium injection,
when false-positive cases may occur [29]. Contrast
material in the collecting system during the excretory-
phase study can give a false impression of an abnormal
nephrogram from streak artefacts, particularly when
using non-ionic contrast material [30]. If helical CT is
available, at least the generalized nephrographic and c
excretory phase studies should be performed [29].
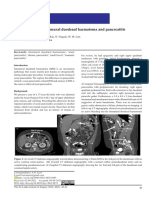
Fig. 1. Acute pyelonephritis in a 31-year-old woman. a, Contrast
However, the excretory phase alone is adequate for material-enhanced helical CT scan obtained during the early
management purposes and in institutions where helical corticomedullary differentiation phase reveals a focal cortical area
CT is unavailable. of minimally decreased attenuation in the upper pole of the left
Sometimes a further delayed CT scan is helpful in kidney with outer bulging (arrow). b, A CT scan obtained during the
generalized homogeneous nephrographic phase shows a focal area
differentiating tumour from an in¯ammatory mass. In
of diminished contrast enhancement extending from the medulla to
this situation, delayed CT studies may show persistent the cortex (arrow). c, The lesion becomes well de®ned on the
excretory phase scan.
# 2000 BJU International, 86 Suppl. 1, 70±79
72 A. KAWASHIMA et al.
Fig. 2. Acute pyelonephritis in a 58-year-
old man with impaired renal function. An
axial view of a fast spin-echo inversion-
recovery pulse sequence (TR/TE/IR: 4800/
15/160) after the IV administration of
gadolinium reveals a large (straight arrow)
and small (curved arrow) focal area of
increased signal intensity in the right
kidney. The remaining right renal
parenchyma shows heterogeneous signal
intensity. Areas of acute pyelonephritis
appear hyper-intense. Note a normal left
kidney with homogeneously low signal
intensity.
contrast enhancement in areas where there was presence of interstitial oedema within the affected renal
previously diminished enhancement after contrast tissue. This process also results in loss of the normal
medium [31]. Delayed CT obtained o3 h after IV corticomedullary junction differentiation on the ultra-
contrast medium administration has been reported to sonogram. In a recent review of 12 patients with acute
be more useful in evaluating the extent of infection than pyelonephritis assessed using power and colour Doppler
are early phase scans [32,33]. Three patterns of delayed US, and enhanced helical CT, power Doppler US was
contrast staining have been described: (i) a nephrogram shown to be superior to colour Doppler US in de®ning the
replacing a variable portion of the decreased attenuation extent of hypoperfusion caused by infection, but inferior
present on early enhanced scans; (ii) a focal area or rim of to helical CT [36].
contrast enhancement surrounding an abscess; and (iii) Excretory urography plays a minor role in imaging the
uncommonly, delayed contrast staining far from the kidneys in patients with acute renal infection, although it
lesions detected on early scans [32,33]. is often requested to screen for urinary obstruction. If
Other CT signs of infection include focal or global used when CT is not readily available, nephrotomogra-
enlargement of the kidney, obliteration of the renal sinus phy after IV contrast administration should be obtained.
and perinephric fat planes, thickening of Gerota's fascia, About 75% of all affected patients have normal urograms
and calyceal effacement caused by swelling of adjacent [19,37]. If the investigation is limited to those patients
renal parenchyma and thickening of the pelvicalyceal who do not respond to appropriate antibiotic therapy
wall [8±10]. Soft-tissue attenuation ®lling defects in the 72 h after initiating treatment, there are signi®cantly
collecting system may represent in¯ammatory debris, more patients with urographic ®ndings that have
blood clots or sloughed tissue from papillary necrosis. immediate clinical signi®cance. Excretory urographic
Uncomplicated acute pyelonephritis in adults had been ®ndings in acute pyelonephritis include renal enlarge-
thought not to cause signi®cant permanent anatomical ment, compression of the collecting system, delayed
or physiological sequelae. Occasionally, severe cases of contrast medium excretion, and diminished contrast
the disease result in papillary necrosis and renal medium concentration. Urography is considered neces-
parenchymal scarring. More recent data suggest that sary to diagnose papillary necrosis, especially in the
new scars may eventually appear on CT in up to half of setting of a diabetic patient with haematuria [21].
the patients who have acute infections with or without Fraser et al. [38] showed that CT was almost as
abscess formation [34]. In moderate and severe cases of sensitive as cortical scintigraphy in detecting focal
infection, nephrographic abnormalities may be present abnormalities in adult patients with the clinical and
for several weeks to months, well after the clinical laboratory diagnosis of acute pyelonephritis. Focal areas
symptoms and laboratory ®ndings have returned to of decreased uptake on renal cortical scans are not
normal [12,35]. It is important not to confuse such speci®c for acute pyelonephritis and may represent
residual changes with ongoing disease requiring con- abscess, infarct, cyst, or tumour.
tinued therapy. With recent improvements in coil technology and the
Ultrasonography, in our experience, is insensitive for introduction of fast-imaging techniques, there have been
evaluating renal infection compared with enhanced CT; promising results with MRI for evaluating acute renal
US may detect acute pyelonephritis as a hypoechoic area infection both experimentally [39] and clinically in
or occasionally a hyperechoic area. This is caused by the children [40,41] (see next section). Clinical experience
# 2000 BJU International, 86 Suppl. 1, 70±79
IMAGING IN ACUTE RENAL INFECTION 73
and laboratory ®ndings cannot reliably differentiate
upper from lower tract infection [45].
In childhood, imaging is indicated with the ®rst
documented UTI in all boys and in all girls <5 years
old; all older girls with febrile or recurrent UTIs should
also be evaluated [42]. Conventional or radionuclide
voiding cysto-urethrography is usually indicated to
determine the presence and degree of re¯ux once the
patient has been adequately treated with antibiotics.
Cortical scintigraphy is more sensitive than urography
or US for detecting acute pyelonephritis and renal scarring
[46±53]. Differential renal function can be assessed
quantitatively by a renal scan; 99mTc-DMSA or 99mTc-
a glucoheptonate are the agents used most often. Cortical
scintigraphy will detect focal or global areas of decreased
uptake of tracer, preserving the renal contour. There may
also be swelling of the kidney corresponding to the areas of
acute in¯ammation. Renal cortical abnormalities are
present in 50±91% of febrile children with UTI but most of
these patients have no detectable VUR [45,50]. When
VUR is present, renal cortical scintigraphic ®ndings are
abnormal in 79±86% of the kidneys in patients with
evidence of VUR overall and in all patients with moderate
or severe re¯ux (grades III±V) [45,50].
Ultrasonography is not invasive and is painless; it is as
sensitive as excretory urography for detecting signi®cant
structural abnormalities of the urinary tract in children
[42]. Children requiring hospitalization for febrile infec-
b tions should at least be evaluated for possible urinary
obstruction with US [42]. Typical US ®ndings of acute
Fig. 3. Acute pyelonephritis in an 8-month-old boy. a, A long-
pyelonephritis include focal or global areas of decreased
itudinal view on a grey-scale ultrasonogram of the right kidney
reveals focal swelling of the upper pole (M). Note the loss of or occasionally increased echogenicity (Fig. 3a), oblit-
corticomedullary differentiation. b, Decreased perfusion (M) visible eration of corticomedullary differentiation, and thicken-
on the power Doppler ultrasonogram. ing of the wall of the renal pelvis [54]. Doppler (colour or
power) US shows areas of decreased or occasionally
in adults is currently limited. In our experience, MRI is a increased perfusion (Fig. 3b) [54,55]. Doppler US can be
useful tool in evaluating adult patients with suspected considered as a possible alternative to CT. In one study,
acute pyelonephritis in whom iodinated contrast media Doppler US showed renal parenchymal abnormalities in
are contraindicated (Fig. 2). However, the cost is a 17 of 22 patients with acute pyelonephritis con®rmed by
signi®cant issue. CT [55]. In a recent study using renal cortical
scintigraphy as the gold standard, Doppler US was
superior to grey-scale US for the diagnosis of acute
Acute pyelonephritis in children
pyelonephritis [54]. Doppler US had a sensitivity of 20%,
Acute pyelonephritis in children is often associated with a speci®city of 97%, a positive predictive value of 96%,
VUR and represents the most severe type of UTI. The and a negative predictive value of 30%, while grey-scale
in¯ammatory changes of acute pyelonephritis are US had corresponding values of 46%, 87%, 89% and 41%
reversible and cause no renal scarring in most cases. [54]. In the same study, the abnormal Doppler US
However, in others acute pyelonephritis results in ®ndings helped to predict future scarring with a positive
irreversible renal scarring [42] which can subsequently predictive value of 85.7% and a negative predictive value
lead to hypertension and chronic renal failure [43]. of 37.2% [54]. However, negative results on grey-scale
Prompt treatment with appropriate antibiotics can and Doppler US do not preclude the diagnosis of acute
minimize or even prevent parenchymal scarring [44]. pyelonephritis [48,51±54].
Imaging plays a much greater role in the diagnosis of Pennington et al. [39] showed that gadolinium-
pyelonephritis in children than in adults as the clinical enhanced fast spin-echo inversion-recovery MRI was
# 2000 BJU International, 86 Suppl. 1, 70±79
74 A. KAWASHIMA et al.
85±92% sensitive and 94±95% speci®c in detecting Urine culture in patients with renal abscess can be
experimentally induced acute pyelonephritis in piglets. negative in 15±20% [15]. Perinephric abscess can extend
More recently, cortical scintigraphy, spiral CT and MRI through Gerota's fascia to the pararenal space (pararenal
were equally sensitive and reliable for detecting acute abscess outside Gerota's fascia). Abscesses may involve
pyelonephritis, but power Doppler US was signi®cantly the psoas muscle and may extend to the pelvis and groin.
less accurate [56]. In 37 paediatric patients with Extrarenal infection in the chest and abdomen (e.g.
suspected acute pyelonephritis, gadolinium-enhanced diverticulitis, pancreatitis) may also extend into the
MRI was superior to renal scintigraphy; MRI detected perinephric space. Perinephric and pararenal abscess
more lesions in 78% and had better interobserver may develop as a complication of xanthogranulomatous
agreement than renal cortical scintigraphy, which pyelonephritis.
detected abnormalities in 68% [40]. MRI has better Excretory urography may occasionally show a renal
spatial resolution than cortical scintigraphy. In another mass with reduced excretion associated with poorly
clinical study, MRI detected lesions of acute pyelo- opaci®ed or effaced adjacent calyces, or may show a
nephritis in two of four patients and areas of scarring localized cortical bulge when the abscess is large and in
in all four [41]. In children, when a complication of the cortex [37]. Depending on the location of the abscess
pyelonephritis is suspected, CT remains the imaging within the kidney, the pelvicalyceal system may be
study of choice, despite the radiation exposure. compressed, displaced, or normal. Perinephric abscesses
may obscure or enlarge the renal contour and may
displace the kidney.
Septic emboli
On US, a typical renal abscess appears as a hypo-echoic
One cause of acute renal infection in adults is or anechoic complex mass with increased transmission of
haematogenous seeding (septic emboli) of the kidney as sound (Fig. 4a). The borders become increasingly well
the result of IV drug abuse, subacute bacterial endocar- de®ned as the abscess encapsulates. Internal echoes that
ditis, or a distant primary focus (e.g. tooth abscess, move with changing position of the patient represent
respiratory tract infection) [5,35]. More than 90% of debris within the abscess cavity. Unfortunately, some
septic emboli are caused by Staphylococcus aureus and abscesses that are obvious on CT fail to show the expected
Streptococcus spp. Haematogenous infection begins in the characteristics on US of a ¯uid-containing mass [35]. For
renal cortex and usually appears as multiple, peripherally example, an abscess may appear as a hypoechoic, poorly
located lesions. Typical CT ®ndings are small wedge- margined mass with scattered low-amplitude echoes and
shaped or rounded areas of hypo-attenuation in the renal poor transmission of sound [14], appearances which also
cortex. When septic foci of infection coalesce and spread occur in focal acute pyelonephritis with no abscess. Serial
into the renal parenchyma, it may not be possible to ultrasonograms can be used to follow this type of lesion,
distinguish ascending and haematogenous infection but CT will usually provide much more useful informa-
[2,12]. Cortical abscesses may be found and associated tion. In a series of patients assessed by Soulen et al. [14],
with perirenal extension of the abscess [5]. The CT US failed to detect seven of 15 intrarenal and extrarenal
features of typical cortical abscesses include a rim of abscesses revealed by CT.
contrast enhancement at the periphery of the hypodense CT is currently the most accurate modality for
lesion. Ancillary CT ®ndings in the lung, spleen, muscle, detecting and following renal abscesses [6,14,22]. An
and skeleton are also indicative of septic emboli. abscess usually appears as a well-de®ned low-density
mass (Fig. 4b). An irregular and thick wall or pseudo-
capsule is better imaged after contrast enhancement
Renal, perirenal (perinephric) and pararenal
[6,34]; the lique®ed purulent material does not enhance.
abscess
Gas (CT numbers of fx150 HU) density in a cystic
Severe in¯ammation may cause multiple small suppura- mass that is ¯uid-®lled (CT numbers of t 10 HU)
tive foci which coalesce into a larger focal collection of strongly suggests abscess formation [57]. Renal par-
pus (with or without a discrete surrounding reparative enchyma around the abscess that appears hypodense on
type of `wall'). This latter complication is by de®nition an early scans may appear hyperdense on delayed views.
acute renal abscess. Perinephric abscess (abscess within Perinephric abscesses appear as soft tissue and/or of ¯uid
the perirenal `Gerota's' fascia) may result from rupture of density within the perirenal space (Fig. 4b). Abscesses
a renal abscess into the perirenal space, but most often may involve the psoas muscle and extend to the iliac
develops directly from acute pyelonephritis. Diabetic fossa and groin. Pararenal abscesses appear similar
patients with calculi and patients with septic emboli except for location and may or may not be associated
are at greater risk of developing abscess. Diabetes mellitus with perinephric or renal abscesses. Gas may occasion-
is found in 75% of patients with perinephric abscess. ally be present.
# 2000 BJU International, 86 Suppl. 1, 70±79
IMAGING IN ACUTE RENAL INFECTION 75
a b
Fig. 4. Renal abscess with extensive retroperitoneal and pelvic extension in a 42-year-old man. a, Longitudinal ultrasonogram of the right
kidney shows a 3r4 cm complex cystic mass with irregular wall and septum (arrows). Note the hypoechoic layer posterior to the right kidney
(arrowheads). b, Enhanced helical CT scan shows a thick-walled cystic mass (arrow) in the right kidney, which is displaced by large right
perinephric and pararenal ¯uid collections involving the right psoas muscle (P). (reprinted with permission from [8]).
Occasionally, a renal abscess may mimic a necrotic perirenal tissues in up to half of patients, and no function
carcinoma as both lesions may have a ¯uid centre and a in the affected kidneys in 45% [60]. The gas collections
thickened or irregular wall which enhances after contrast can often go unrecognized without nephrotomography.
medium. The patient's symptoms usually clarify the CT is the best modality for detecting the presence of gas
diagnosis injection. However, in some patients percuta- and for de®ning the extent of the disease [57,58,60,64±
neous aspiration and drainage may be indicated, and 66]. CT also detects gas within the renal parenchyma or
cytological examination of the ¯uid may be needed. in the subcapsular, perinephric and/or pararenal space,
Rarely, a post-in¯ammatory cystic ¯uid collection may in the collecting system, or occasionally in the vascular
develop in the area of acute pyelonephritis during or after system (Fig. 5).
the course of antibiotic treatment. These collections are The typical US appearance of gas-producing renal
usually small (<3 cm in diameter) with no appreciable infections is of markedly echogenic areas within the renal
wall thickening or contrast enhancement. However, CT- parenchyma or perirenal tissues, with distal shadowing
guided needle aspiration may be required to exclude an containing low-level echoes and reverberations [57].
abscess, if the ¯uid collection does not resolve. Differentiation from renal calculi may be dif®cult. The
affected kidney may be completely obscured by the gas
and a plain ®lm should be obtained to differentiate air
Emphysematous pyelonephritis
from calculi.
Emphysematous pyelonephritis is a fulminant gas-form- MRI is of limited use for detecting gas in the urinary
ing infection of the renal parenchyma [58±60]. Diabetes tract. Gas appears as an area of signal void which cannot
mellitus is present in 85±100% of the patients and is be differentiated from calculi, renal calci®cation and
often uncontrolled. Most patients present with symptoms ¯owing blood on T1- and T2-weighted spin-echo images
of severe acute pyelonephritis, urosepsis, or shock, and [57]. Concentrated gadolinium contrast agents excreted
calculi may be present. Escherichia coli, Klebsiella into the collecting system also appear as signal void
pneumonia and Proteus mirabilis are the most common because of the T2 shortening effect.
organisms. Emphysematous pyelonephritis in the trans- Renal scintigraphy has been used to assess renal
planted kidney has been reported [61,62]. Gas in function and especially to evaluate the uninvolved
emphysematous UTI has been attributed to mixed acid kidney before surgery, but otherwise it is of little value
fermentation of tissue glucose by gas-forming organisms in emphysematous pyelonephritis.
[59]. Gas can be limited to the renal collecting system, In recent studies by Wan et al. [66,67], acute gas-
the ureter, and the bladder. These entities are distinct forming bacterial infection of the kidneys was classi®ed
from true emphysematous pyelonephritis and have a into two types based on CT and plain-®lm features.
better prognosis [63]. Classic emphysematous pyelonephritis (type I), charac-
Excretory urography may show mottled or crescent- terized by parenchymal destruction, the presence of
shaped gas collections in the renal parenchyma and streaky or mottled gas, and little or no ¯uid (Fig. 4), had a
# 2000 BJU International, 86 Suppl. 1, 70±79
76 A. KAWASHIMA et al.
Fig. 5. Emphysematous pyelonephritis in
a 62-year-old woman with poorly
controlled diabetes mellitus. The
unenhanced CT scan shows the enlarged
left kidney with gas in the renal
parenchyma, renal pelvis (curved arrow)
and perirenal space (open arrow). Note the
gas in the left renal vein and inferior vena
cava (large straight arrows) and
thickening of Gerota's fascia on the left
(arrowheads). Calculi are present in the
collecting system bilaterally (small straight
arrows).
worse prognosis than renal infection characterized by cases, pyonephrosis is indistinguishable from an unin-
either renal or perirenal ¯uid collections with bubbly or fected hydronephrosis. The nephrogram of uncomplicated
loculated gas in the parenchyma or in the collecting acute obstruction may have ®ne striations, or a prolonged
system (type II). The authors speculate that the latter corticomedullary differentiation phase, and subsequently
more favourable group is still capable of an immune a prolonged homogeneous nephrogram. Conversely, acute
response. pyelonephritis may show a coarsely striated nephrogram.
The presence of an abnormal nephrogram (especially if
there is focal bulging of the renal contour) in the
Pyonephrosis
parenchyma of an obstructed kidney suggests super-
Infected hydronephrosis (pyonephrosis) is usually con- imposed infection rather than uncomplicated hydro-
sidered to be a urological emergency requiring urgent nephrosis. Unfortunately, renal parenchymal changes
drainage. Urinary decompression and drainage may be are not often present. CT can also detect thickened walls of
accomplished by either retrograde stent placement or by the pelvis and ureter, ¯uid-¯uid levels (pus-urine, urine-
percutaneous aspiration and nephrostomy. Pyonephrosis debris, or contrast-debris levels), and gas within the
results from acute or more frequently chronic obstruction collecting system. Clinical ®ndings are important in
and superimposed UTI [11,68,69]. Associated parench- differentiating hydronephrosis from pyonephrosis.
ymal involvement ranges from uncomplicated pyelone- Suppurative renal infection in patients with chronic
phritis to extensive destruction. urinary obstruction occasionally results in spontaneous
If suf®cient renal function is present, excretory reno-colic ®stula formation [75].
urography shows urinary tract obstruction with absent
or delayed opaci®cation of the collecting system, renal
Infected cyst
enlargement, and occasionally a hyperdense nephro-
gram on delayed ®lms [69]. Occasionally a renal cyst may be secondarily infected by
Ultrasonography may be indicated to seek evidence of haematogenous dissemination, re¯ux, surgical manip-
urinary obstruction in patients with acute renal infection ulation, or cyst puncture. The clinical diagnosis of
who do not improve with antibiotics. However, in acute superimposed infection of a renal cyst is dif®cult because
obstruction, caliectasis may be minimal, which renders typically speci®c symptoms and pyuria are absent. It is
US less reliable if the diagnosis of obstruction is based on dif®cult to differentiate an infected simple renal cyst from
pelvicalyceal dilatation alone. Adding resistive index (RI) an abscess both clinically and radiologically [35,76,77].
measurements with pulsed Doppler US may enhance the An infected cyst may also mimic a renal cyst complicated
sensitivity of the test in diagnosing obstruction, but there by intracystic haemorrhage on imaging studies.
have been no studies to examine its speci®city in patients Percutaneous cyst aspiration and drainage under CT or
with infection [2]. The value of the RI is extremely US guidance may be indicated for diagnosis and
controversial. US may show echogenic material in the treatment.
dilated pelvicalyceal system, sometimes with a urine-
debris level, but often the features are those of simple
Acute fungal infection
hydronephrosis [70±73].
CT is helpful in identifying hydronephrosis and deter- Patients with acute leukaemia and neutropenia are
mining the level and cause of obstruction [35,74]. In most susceptible to haematogenously disseminated fungal
# 2000 BJU International, 86 Suppl. 1, 70±79
IMAGING IN ACUTE RENAL INFECTION 77
infections that may seed the kidneys with multiple small 5 Goldman SM. Acute and chronic urinary infection: present
abscesses [78,79]. The most common fungal infection is concepts and controversies. Urol Radiol 1988; 10: 17±24
Candida. CT typically shows multiple small hypodense 6 Hoddick W, Jeffrey RB, Goldberg HI, Federle MP, Laing FC.
lesions in the spleen, liver and kidneys. Enhanced CT is CT and sonography of severe renal and perirenal infections.
Am J Roentgenol 1983; 140: 517±20
superior to unenhanced CT in showing the lesions, but
7 June CH, Browning MD, Smith LP et al. Ultrasonography
CT is not as sensitive as a renal biopsy of the kidney in
and computed tomography in severe urinary tract infection.
diagnosing disseminated fungal disease [80].
Arch Intern Med 1985; 145: 841±5
Fungal renal involvement in patients with AIDS is 8 Kawashima A, Sandler CM, Goldman SM, Raval BK,
relatively uncommon [81]. Renal mucormycosis has Fishman EK. CT of renal in¯ammatory disease.
been reported in such patients in Italy. A special Radiographics 1997; 17: 851±66
propensity for vascular invasion can cause extensive 9 Kawashima A, Sandler CM, Ernst RD, Goldman SM, Raval B,
cortical infarcts and medullary necrosis in the kidneys Fishman EK. Renal in¯ammatory disease: the current role of
[82]. CT shows a mixed hypo- and hyperdense pattern in CT. Crit Rev Diagn Imaging 1997; 38: 369±415
the entire kidney. 10 Kawashima A, Sandler CM, Goldman SM. Current roles and
controversies in the imaging evaluation of acute renal
infection. World J Urol 1998; 16: 9±17
Conclusion 11 Lowe LH, Zagoria RJ, Baumgartner BR, Dyer RB. Role of
While renal imaging is not routinely indicated in cases of imaging and intervention in complex infections of the
urinary tract. Am J Roentgenol 1994; 163: 363±7
uncomplicated renal infection, CT is a readily available,
12 Papanicolaou N, P®ster RC. Acute renal infections. Radiol
highly sensitive modality for the diagnosis and manage-
Clin North Am 1996; 34: 965±95
ment of patients with acute renal infection. Excretory 13 Roberts JA. Pyelonephritis, cortical abscess, and perinephric
urography and US are frequently used to screen for abscess. Urol Clin North Am 1986; 13: 637±45
urinary obstruction, renal calculi, and underlying 14 Soulen MC, Fishman EK, Goldman SM, Gatewood OM.
anomalies. CT is of value in establishing the diagnosis Bacterial renal infection: role of CT. Radiology 1989; 171:
in equivocal cases, in evaluating high-risk patients, and 703±7
in determining the nature and extent of disease. Lesions 15 Thornbury JR. Acute renal infections. Urol Radiol 1991; 12:
of acute pyelonephritis can be best shown on CT scans 209±13
obtained during the homogeneous nephrographic phase 16 Davidson AJ, Talner LB. Urographic and angiographic
of contrast enhancement. However, views obtained abnormalities in adult-onset acute bacterial nephritis.
during the excretory phase when there is pelvicalyceal Radiology 1973; 106: 249±56
®lling with contrast medium are less equivocal for 17 Lee JK, McClennan BL, Melson GL, Stanley RJ. Acute focal
bacterial nephritis: emphasis on gray scale sonography
diagnosing renal abscesses.
and computed tomography. Am J Roentgenol 1980; 135:
Cortical scintigraphy is usually the preferred imaging
87±92
study for the evaluation of children with acute pyelo-
18 Rosen®eld AT, Glickman MG, Taylor KJ, Crade M, Hodson J.
nephritis, although power Doppler US can be considered Acute focal bacterial nephritis (acute lobar nephronia).
as a possible alternative. Recent studies have shown that Radiology 1979; 132: 553±61
gadolinium-enhanced MRI is equal to or better than 19 Kanel KT, Kroboth FJ, Schwentker FN, Lecky JW. The
scintigraphy in showing pyelonephritis. intravenous pyelogram in acute pyelonephritis. Arch Intern
Med 1988; 148: 2144±8
20 Rodriguez de Velasquez A, Yoder IC, Velasquez PA,
Papanicolaou N. Imaging the effects of diabetes on the
References genitourinary system. Radiographics 1995; 15: 1051±68
1 Dunnick NR, Sandler CM, Amis ES Jr et al. Renal 21 Sentochnik DE, Eliopoulos GM. Infection and diabetes. In:
in¯ammatory disease. In Dunnick NR, Sandler CM, Amis Kahn CR, Weir GC, eds. Joslin's Diabetes Mellitus, 13th edn.
ES Jr, Newhouse JH, eds. Textbook of Uroradiology. Baltimore: Philadelphia: Lea & Febiger, 1994: 867±88
Williams & Wilkins, 1996: 163±89 22 Rauschkolb EN, Sandler CM, Patel S, Childs TL. Computed
2 Talner LB, Davidson AJ, Lebowitz RL, Dalla Palma L, tomography of renal in¯ammatory disease. J Comput Assist
Goldman SM. Acute pyelonephritis: can we agree on Tomogr 1982; 6: 502±6
terminology? Radiology 1994; 192: 297±305 23 Rigsby CM, Rosen®eld AT, Glickman MG, Hodson J.
3 Huang JJ, Sung JM, Chen KW, Ruaan MK, Shu GH, Chuang Hemorrhagic focal bacterial nephritis: ®ndings on gray-
YC. Acute bacterial nephritis: a clinicoradiologic correlation scale sonography and CT. Am J Roentgenol 1986; 146:
based on computed tomography. Am J Med 1992; 93: 289±98 1173±7
4 Zaontz MR, Pahira JJ, Wolfman M, Gargurevich AJ, Zeman 24 Gold RP, McClennan BL, Rottenberg RR. CT appearance of
RK. Acute focal bacterial nephritis: a systematic approach to acute in¯ammatory disease of the renal interstitium. Am J
diagnosis and treatment. J Urol 1985; 133: 752±7 Roentgenol 1983; 141: 343±9
# 2000 BJU International, 86 Suppl. 1, 70±79
78 A. KAWASHIMA et al.
25 Gold RP, McClennan BL, Kenney PJ et al. Acute infections of pyelonephritis in childhood: 27 year follow up. Br Med J
the renal parenchyma. In Pollack HM, ed. Clinical 1989; 299: 703±6
Urography. Philadelphia: WB Saunders Co., 1990: 799±821 44 Benador D, Benador N, Slosman D, Mermillod B, Girardin E.
26 Hill GS. Renal infection. In Hill GS, ed. Uropathology. New Are younger children at highest risk of renal sequelae after
York: Churchill Livingstone, 1989 pyelonephritis? Lancet 1997; 349: 17±9
27 Saunders HS, Dyer RB, Shifrin RY, Scharling ES, Bechtold 45 Majd M, Rushton HG, Jantausch B, Wiedermann BL.
RE, Zagoria RJ. The CT nephrogram: implications for Relationship among vesicoureteral re¯ux, P-®mbriated
evaluation of urinary tract disease. Radiographics 1995; Escherichia coli, and acute pyelonephritis in children with
15: 1069±85 febrile urinary tract infection. J Pediatr 1991; 119: 578±85
28 Kawashima A, Fishman EK, Goldman SM, Sandler CM. 46 Risdon RA, Godley ML, Parkhouse HF, Gordon I, Ransley
Helical CT of acute pyelonephritis: is there an ideal timing PG. Renal pathology and the 99mTc-DMSA image during
for imaging? Radiology 1996; 201: 148±9 the evolution of the early pyelonephritic scar: an experi-
29 Kawashima A, Sandler CM, Ernst RD, Takahashi N, mental study. J Urol 1994; 151: 767±73
Fishman EK, Goldman SM. Multiphasic contrast-enhanced 47 Rushton HG, Majd M, Chandra R, Yim D. Evaluation of 99m
CT of acute renal infection. Radiology 1998; 209: 337 technetium-dimercapto-succinic acid renal scans in experi-
30 Sussman SK, Illescas FF, Opalacz JP, Yirga P, Foley LC. mental acute pyelonephritis in piglets. J Urol 1988; 140:
Renal streak artifact during contrast-enhanced CT. 1169±74
Comparison of low versus high osmolality contrast media. 48 Verber IG, Strudley MR, Meller ST. 99mTc dimercaptosuc-
Abdom Imaging 1993; 18: 180±5 cinic acid (DMSA) scan as ®rst investigation of urinary tract
31 Ishikawa I, Saito Y, Onouchi Z et al. Delayed contrast infection. Arch Dis Child 1988; 63: 1320±5
enhancement in acute focal bacterial nephritis: CT features. 49 Majd M, Rushton HG. Renal cortical scintigraphy in the
J Comput Assist Tomogr 1985; 9: 894±7 diagnosis of acute pyelonephritis. Semin Nucl Med 1992; 22:
32 Dalla-Palma L, Pozzi-Mucelli F, Pozzi-Mucelli RS. Delayed 98±111
CT ®ndings in acute renal infection. Clin Radiol 1995; 50: 50 Rushton HG, Majd M. Dimercaptosuccinic acid renal
364±70 scintigraphy for the evaluation of pyelonephritis and
33 Dalla-Palma L, Pozzi-Mucelli R, Pozzi-Mucelli F. Delayed CT scarring: a review of experimental and clinical studies.
in acute renal infection. Semin Ultrasound CT MR 1997; 18: J Urol 1992; 148: 1726±32
122±8 51 Melis K, Vandevivere J, Hoskens C, Vervaet A, Sand A, Van
34 Soulen MC, Fishman EK, Goldman SM. Sequelae of acute Acker KJ. Involvement of the renal parenchyma in acute
renal infections: CT evaluation. Radiology 1989; 173: 423±6 urinary tract infection: the contribution of 99mTc dimer-
35 Goldman SM, Fishman EK. Upper urinary tract infection: captosuccinic acid scan. Eur J Pediatr 1992; 151: 536±9
the current role of CT, ultrasound, and MRI. Semin 52 Kass EJ, Fink-Bennett D, Cacciarelli AA, Balon H, Pavlock S.
Ultrasound CT MR 1991; 12: 335±60 The sensitivity of renal scintigraphy and sonography in
36 Clautice-Engle T, Jeffrey RB Jr. Renal hypoperfusion: value detecting nonobstructive acute pyelonephritis. J Urol 1992;
of power Doppler imaging. Am J Roentgenol 1997; 168: 148: 606±8
1227±31 53 Sreenarasimhaiah V, Alon US. Uroradiologic evaluation of
37 Silver TM, Kass EJ, Thornbury JR, Konnak JW, Wolfman children with urinary tract infection: are both ultrasono-
MG. The radiological spectrum of acute pyelonephritis in graphy and renal cortical scintigraphy necessary? J Pediatr
adults and adolescents. Radiology 1976; 118: 65±71 1995; 127: 373±7
38 Fraser IR, Birch D, Fairley KF et al. A prospective study of 54 Jequier S, Jequier JC, Hanquinet S. Acute childhood
cortical scarring in acute febrile pyelonephritis in adults: pyelonephritis: predictive value of positive sonographic
clinical and bacteriological characteristics. Clin Nephrol ®ndings in regard to later parenchymal scarring. Acad Radiol
1995; 43: 159±64 1998; 5: 344±53
39 Pennington DJ, Lonergan GJ, Flack CE, Waguespack RL, 55 Dacher JN, P®ster C, Monroc M, Eurin D, LeDosseur P.
Jackson CB. Experimental pyelonephritis in piglets: diag- Power Doppler sonographic pattern of acute pyelonephritis
nosis with MR imaging. Radiology 1996; 201: 199±205 in children: comparison with CT. Am J Roentgenol 1996;
40 Lonergan GJ, Pennington DJ, Morrison JC, Haws RM, 166: 1451±5
Grimley MS, Kao TC. Childhood pyelonephritis: comparison 56 Majd M, Shalaby-Rana E, Markle B et al. Diagnosis of
of gadolinium-enhanced MR imaging and renal cortical experimental acute pyelonephritis in piglets: comparison of
scintigraphy for diagnosis. Radiology 1998; 207: 377±84 99mTc-DMSA SPECT, spiral CT, MRI, and power Doppler
41 Poustchi-Amin M, Leonidas JC, Palestro C, Hassankhani A, sonography. Presented at The Society of Uroradiology
Gauthier B, Trachtman H. Magnetic resonance imaging in Annual Meeting. Hamilton, Bermuda 1998
acute pyelonephritis. Pediatr Nephrol 1998; 12: 579±80 57 Joseph RC, Amendola MA, Artze ME et al. Genitourinary
42 Rushton HG. Urinary tract infections in children. tract gas: imaging evaluation. Radiographics 1996; 16: 295±
Epidemiology, evaluation, and management. Pediatr Clin 308
North Am 1997; 44: 1133±69 58 Ahlering TE, Boyd SD, Hamilton CL et al. Emphysematous
43 Jacobson SH, Eklof O, Eriksson CG, Lins LE, Tidgren B, pyelonephritis: a 5-year experience with 13 patients. J Urol
Winberg J. Development of hypertension and uraemia after 1985; 134: 1086±8
# 2000 BJU International, 86 Suppl. 1, 70±79
IMAGING IN ACUTE RENAL INFECTION 79
59 Huang JJ, Chen KW, Ruaan MK. Mixed acid fermentation of 73 Subramanyam BR, Raghavendra BN, Bosniak MA, Le¯eur
glucose as a mechanism of emphysematous urinary tract RS, Rosen RJ, Horii SC. Sonography of pyonephrosis: a
infection. J Urol 1991; 146: 148±51 prospective study. Am J Roentgenol 1983; 140: 991±3
60 Michaeli J, Mogle P, Perlberg S, Heiman S, Caine M. 74 Fultz PJ, Hampton WR, Totterman SM. Computed tomo-
Emphysematous pyelonephritis. J Urol 1984; 131: 203±8 graphy of pyonephrosis. Abdom Imaging 1993; 18: 82±7
61 Balsara VJ, Raval B, Maklad NF. Emphysematous pyelone- 75 Parvey HR, Cochran ST, Payan J, Goldman S, Sandler CM.
phritis in a renal transplant: sonographic and computed Renocolic ®stulas: complementary roles of computed
tomographic features. J Ultrasound Med 1985; 4: 97±9 tomography and direct pyelography. Abdom Imaging
62 Potter JL, Sullivan BM, Flournoy JG, Gerza C. Emphysema in 1997; 22: 96±9
the renal allograft. Radiology 1985; 155: 51±2 76 Feldberg MA, Mali WP. An infected renal cyst. Urol Radiol
63 Evanoff GV, Thompson CS, Foley R, Weinman EJ. Spectrum 1980; 2: 47±9
of gas within the kidney. Emphysematous pyelonephritis 77 Frishman E, Orron DE, Heiman Z, Kessler A, Kaver I, Graif
and emphysematous pyelitis. Am J Med 1987; 83: 149±54 M. Infected renal cysts: sonographic diagnosis and manage-
64 Kim DS, Woesner ME, Howard TF, Olson LK. ment. J Ultrasound Med 1994; 13: 7±10
Emphysematous pyelonephritis demonstrated by computed 78 Kuhlman JE. Renal and urinary tract complications. In:
tomography. Am J Roentgenol 1979; 132: 287±8 Kuhlman JE, ed. CT of the Immunocompromised Host. New
65 Lautin EM, Gordon PM, Friedman AC, Dourmashkin L, York: Churchill Livingstone, 1991: 89±114
Fromowitz F. Emphysematous pyelonephritis: optimal 79 Spring DB. Fungal diseases of the urinary tract. In: Pollack
diagnosis and treatment. Urol Radiol 1979; 1: 93±6 HM, ed. Clinical Urography, 1st edn. Philadelphia: WB
66 Wan YL, Lee TY, Bullard MJ, Tsai CC. Acute gas-producing Saunders, 1990: 987±98
bacterial renal infection: correlation between imaging 80 Shirkhoda A. CT ®ndings in hepatosplenic and renal
®ndings and clinical outcome. Radiology 1996; 198: 433±8 candidiasis. J Comput Assist Tomogr 1987; 11: 795±8
67 Wan YL, Lo SK, Bullard MJ, Chang PL, Lee TY. Predictors of 81 Vaziri ND, Barbari A, Licorish K, Cesario T, Gupta S.
outcome in emphysematous pyelonephritis. J Urol 1998; Spectrum of renal abnormalities in acquired immune±
159: 369±73 de®ciency syndrome. J Natl Med Assoc 1985; 77: 369±75
68 Kenney PJ, Breatnach ES, Stanley RJ. Pyonephrosis. In: 82 Vesa J, Bielsa O, Arango O, Llado C, Gelabert A. Massive
Pollack HM, ed. Clinical Urography. Philadelphia: WB renal infarction due to mucormycosis in an AIDS patient.
Saunders, 1990: 843±9 Infection 1992; 20: 234±6
69 Yoder IC, P®ster RC, Lindfors KK, Newhouse JH.
Pyonephrosis: imaging and intervention. Am J Roentgenol
1983; 141: 735±40 Authors
70 Colemen BG, Arger PH, Mulhern CB Jr, Pollack HM, Banner A. Kawashima, MD, Associate Professor.
MP. Pyonephrosis: sonography in the diagnosis and C.M. Sandler, MD, Professor and Vice Chairman.
management. Am J Roentgenol 1981; 137: 939±43 S.M. Goldman, MD, Professor and Chairman.
71 Jeffrey RB, Laing FC, Wing VW, Hoddick W. Sensitivity of Correspondence: A. Kawashima, MD, Department of Radiology,
sonography in pyonephrosis: a reevaluation. Am J The University of Texas-Houston Medical School, Lyndon B.
Roentgenol 1985; 144: 71±3 Johnson General Hospital, 5656 Kelley Street, Houston, Texas
72 Nicolet V, Carignan L, Dubuc G, Hebert G, Bourdon F, 77026, USA.
Paquin F. Thickening of the renal collecting system: a e-mail: akira.kawashima@uth.tmc.edu
nonspeci®c ®nding at US. Radiology 1988; 168: 411±3
# 2000 BJU International, 86 Suppl. 1, 70±79
You might also like
- Risk Adjustment Coding PDFDocument38 pagesRisk Adjustment Coding PDFGordana Puzovic100% (2)
- AEMT Medications: Drug Card InformationDocument2 pagesAEMT Medications: Drug Card InformationAlex Lupi50% (2)
- Anthropology in NursingDocument6 pagesAnthropology in Nursingsoften100% (5)
- AP NAMACB Reference Guide - 2021-11-02Document57 pagesAP NAMACB Reference Guide - 2021-11-02Regina RodriguesNo ratings yet
- Assignment ON: Writing The Reference and BibliographyDocument8 pagesAssignment ON: Writing The Reference and BibliographyAru Verma50% (2)
- Necrotizing Pancreatitis - DBarilDocument25 pagesNecrotizing Pancreatitis - DBarilataner1991No ratings yet
- Antibiotic Mixing Chart With SAMF InfoDocument8 pagesAntibiotic Mixing Chart With SAMF Infosumayyah995No ratings yet
- Test-4 Angina Pectoris Text ADocument11 pagesTest-4 Angina Pectoris Text ANaveen Abraham100% (2)
- Genitourinary Imaging in ChildrenDocument18 pagesGenitourinary Imaging in ChildrenmariakjaimesNo ratings yet
- Renal and Perinephric AbscessDocument11 pagesRenal and Perinephric AbscessYusak DpNo ratings yet
- Open Access Journal of Urology & Nephrology: Interventional Radiology Techniques in The Genitourinary TractDocument5 pagesOpen Access Journal of Urology & Nephrology: Interventional Radiology Techniques in The Genitourinary Tractalfredo elizondoNo ratings yet
- E tratamentPA PDFDocument7 pagesE tratamentPA PDFPavel SebastianNo ratings yet
- PylonefritisDocument8 pagesPylonefritissintaNo ratings yet
- 02 02 Original PyonephrosisDocument4 pages02 02 Original PyonephrosisArief MunandharNo ratings yet
- Ten Tips To Manage Severe Acute Pancreatitis in An Intensive Care UnitDocument4 pagesTen Tips To Manage Severe Acute Pancreatitis in An Intensive Care UnitRicardo Victor PereiraNo ratings yet
- Renal Tuberculosis Mimicking Renal Cell CarcinomaDocument3 pagesRenal Tuberculosis Mimicking Renal Cell CarcinomaNingsih 09No ratings yet
- Case 16589: Anterior Urethra Mucosal Dissection and Contrast Medium Urethro-Venous IntravasationDocument11 pagesCase 16589: Anterior Urethra Mucosal Dissection and Contrast Medium Urethro-Venous IntravasationSaptantoNo ratings yet
- Medicina 57 00032 (01 14)Document14 pagesMedicina 57 00032 (01 14)fauzan nandana yoshNo ratings yet
- Preferred Examination: Right Ureteral Calculus With Periureteral StrandingDocument26 pagesPreferred Examination: Right Ureteral Calculus With Periureteral StrandingErrina YustiraNo ratings yet
- Perinephric and Intranephric Abscesses: Review: The LiteratureDocument8 pagesPerinephric and Intranephric Abscesses: Review: The LiteratureriskyNo ratings yet
- Abdomen Agudo - Radiologic Clinics of Northamerica 2003Document254 pagesAbdomen Agudo - Radiologic Clinics of Northamerica 2003arsilbNo ratings yet
- Article 2 Nephrostomy Technical Indications PDFDocument14 pagesArticle 2 Nephrostomy Technical Indications PDFRoberto AmayaNo ratings yet
- Imaging of Acute Cholecystitis and Cholecystitis-Associated Complications in The Emergency SettingDocument7 pagesImaging of Acute Cholecystitis and Cholecystitis-Associated Complications in The Emergency SettingMita RestuNo ratings yet
- Imaging in PediatricDocument25 pagesImaging in PediatricAlina MedrihanNo ratings yet
- Renal and Perinephric Abscess - UpToDateDocument27 pagesRenal and Perinephric Abscess - UpToDate76q88b4yrxNo ratings yet
- Complicated Pyelonephritis Associated With Chronic Renal Stone DiseaseDocument12 pagesComplicated Pyelonephritis Associated With Chronic Renal Stone Diseasecrisvbarros8865No ratings yet
- Imaging of Acute PancreatitisDocument22 pagesImaging of Acute PancreatitisAde Handayani IINo ratings yet
- Xanthogranulomatous Pyelonephritis: Radiologic Review.: Poster No.: Congress: Type: AuthorsDocument17 pagesXanthogranulomatous Pyelonephritis: Radiologic Review.: Poster No.: Congress: Type: AuthorsChavdarNo ratings yet
- Shear Wave Elastography in Assessment of Liver Stiffness and Prediction of Gastro-Esophageal Varices in Patients With Liver CirrhosisDocument9 pagesShear Wave Elastography in Assessment of Liver Stiffness and Prediction of Gastro-Esophageal Varices in Patients With Liver CirrhosisPriska ForceveeanaNo ratings yet
- Ce (Ra1) F (Ac) Pf1 (Agak) Pfa (Ak) PB (NC Ag) PN (SL)Document3 pagesCe (Ra1) F (Ac) Pf1 (Agak) Pfa (Ak) PB (NC Ag) PN (SL)Anwesa ChakrabortyNo ratings yet
- Fournier'S Gangrene 12.1 Summary of RecommendationsDocument10 pagesFournier'S Gangrene 12.1 Summary of RecommendationsNovi Y'uZzmanNo ratings yet
- Renal Disease in TuberculosisDocument10 pagesRenal Disease in TuberculosismiguelNo ratings yet
- Choledochal Cysts: Part 2 of 3: DiagnosisDocument6 pagesCholedochal Cysts: Part 2 of 3: DiagnosisKarla ChpNo ratings yet
- Diverticulitis: A Comprehensive Review With Usual and Unusual ComplicationsDocument9 pagesDiverticulitis: A Comprehensive Review With Usual and Unusual ComplicationsMerlin MuktialiNo ratings yet
- Sonography First-Line Modality in Diagnosis of Acute Colonic Diverticulitis 2013Document6 pagesSonography First-Line Modality in Diagnosis of Acute Colonic Diverticulitis 2013waldemar russellNo ratings yet
- Current Management of Incidental Gallbladder Cancer - A ReviewDocument7 pagesCurrent Management of Incidental Gallbladder Cancer - A ReviewHugo JBNo ratings yet
- Ureteral Stent For Ureteral Stricture: James F. Borin and Elspeth M. McdougallDocument13 pagesUreteral Stent For Ureteral Stricture: James F. Borin and Elspeth M. McdougallOoNo ratings yet
- Cholecystitis: Kaitlyn J. Kelly and Sharon Marie WeberDocument10 pagesCholecystitis: Kaitlyn J. Kelly and Sharon Marie WeberMasithaNo ratings yet
- Acute Pyelonephritis - Correlation of Clinical Parameter With Radiological Imaging AbnormalitiesDocument7 pagesAcute Pyelonephritis - Correlation of Clinical Parameter With Radiological Imaging AbnormalitiesKhristine Caye FernandezNo ratings yet
- Tuberculous Peritonitis:what About Imaging: Poster No.: Congress: Type: AuthorsDocument9 pagesTuberculous Peritonitis:what About Imaging: Poster No.: Congress: Type: AuthorswurifreshNo ratings yet
- Rui 2007Document5 pagesRui 2007Radiologi RSPDNo ratings yet
- Obstructive Nephropathy As A Result of Malignant Neoplasms: A Single Centre ExperienceDocument4 pagesObstructive Nephropathy As A Result of Malignant Neoplasms: A Single Centre ExperienceTriponiaNo ratings yet
- It Is Not Always A Cholangiocarcinoma: Unusual Peritoneal Carcinomatosis Revealing Gastric AdenocarcinomaDocument5 pagesIt Is Not Always A Cholangiocarcinoma: Unusual Peritoneal Carcinomatosis Revealing Gastric AdenocarcinomaIJAR JOURNALNo ratings yet
- Ultrasonography of The Spleen. Pictorial EssayDocument12 pagesUltrasonography of The Spleen. Pictorial EssayMihaela TancoNo ratings yet
- Silent NightDocument6 pagesSilent NightApril Rae Obregon GarcesNo ratings yet
- Gut05400426 PDFDocument11 pagesGut05400426 PDFCarlos Rodolfo SinibaldiNo ratings yet
- Case Report On Bilateral Emphysematous Pyelonephr - 2024 - International JournalDocument4 pagesCase Report On Bilateral Emphysematous Pyelonephr - 2024 - International JournalRonald QuezadaNo ratings yet
- Minimal Access Retroperitoneal Pancreatic NecrosectomyDocument7 pagesMinimal Access Retroperitoneal Pancreatic NecrosectomyIsrael BlancoNo ratings yet
- Subsidence of Hypertension in A Patient With GiantDocument4 pagesSubsidence of Hypertension in A Patient With GiantSinbijeo GinNo ratings yet
- Aghiz 3Document12 pagesAghiz 3nandaaa aprilNo ratings yet
- Complications of Percutaneous Nephrostomy Tube Placement To Treat NephrolithiasisDocument4 pagesComplications of Percutaneous Nephrostomy Tube Placement To Treat NephrolithiasisPande Made FitawijamariNo ratings yet
- Imaging in Chronic PancreatitisDocument7 pagesImaging in Chronic Pancreatitisdesy 102017135No ratings yet
- CT Findings in Acute Peritonitis: A Pattern-Based ApproachDocument6 pagesCT Findings in Acute Peritonitis: A Pattern-Based ApproachNia FitriyaniNo ratings yet
- Non Perforated Peptic Ulcer Disease, Multidetector CT Finding, Complication, and Differential DiagnosisDocument15 pagesNon Perforated Peptic Ulcer Disease, Multidetector CT Finding, Complication, and Differential DiagnosisIndra PrimaNo ratings yet
- Scrotal Abscess As The First Symptom of Fatal Necrotizing PancreatitisDocument4 pagesScrotal Abscess As The First Symptom of Fatal Necrotizing PancreatitisTeja Laksana NukanaNo ratings yet
- Mesentery, Omentum, Peritoneum: Inflammatory, Infectious Diseases and Pseudo LesionsDocument11 pagesMesentery, Omentum, Peritoneum: Inflammatory, Infectious Diseases and Pseudo LesionsRendra SyaniNo ratings yet
- Diverticuloza DuodenalaDocument4 pagesDiverticuloza Duodenalaraluca77No ratings yet
- Renal BiopsyDocument4 pagesRenal BiopsyDeshan AdikariNo ratings yet
- Portal Vein Thrombosis in Cirrhosis Interventional Treatment OptionsDocument8 pagesPortal Vein Thrombosis in Cirrhosis Interventional Treatment OptionsRisneac EvelinaNo ratings yet
- Small-Bowel and Mesenteric Injuries in Blunt Trauma of The AbdomenDocument8 pagesSmall-Bowel and Mesenteric Injuries in Blunt Trauma of The AbdomenGina Kristina NanginNo ratings yet
- Efficacy and Safety of Early Systemic.14Document5 pagesEfficacy and Safety of Early Systemic.14Dr WittyNo ratings yet
- Pyonefrosis Part 2Document8 pagesPyonefrosis Part 2Kevin MitnickNo ratings yet
- SECTION I Inflammatory, Infective, and Congenital: Pyogenic Liver AbscessDocument12 pagesSECTION I Inflammatory, Infective, and Congenital: Pyogenic Liver AbscessandresNo ratings yet
- Pancreatitis Imaging ApproachDocument19 pagesPancreatitis Imaging ApproachelvaNo ratings yet
- Accuracy of Ultrasonography in The Diagnosis of Acute Calculous Cholecystitis: Review of The LiteratureDocument4 pagesAccuracy of Ultrasonography in The Diagnosis of Acute Calculous Cholecystitis: Review of The LiteratureAnasriNstNo ratings yet
- 8874 33333 1 PBDocument3 pages8874 33333 1 PBRanga PereraNo ratings yet
- Endoscopic Ultrasound Management of Pancreatic Lesions: From Diagnosis to TherapyFrom EverandEndoscopic Ultrasound Management of Pancreatic Lesions: From Diagnosis to TherapyAntonio FacciorussoNo ratings yet
- About Lung Cancer: Overview and TypesDocument13 pagesAbout Lung Cancer: Overview and TypesGordana PuzovicNo ratings yet
- 18dway9Document8 pages18dway9Gordana PuzovicNo ratings yet
- Computed Tomography in The Diagnosis of Subcapsular and Perirenal HematomaDocument6 pagesComputed Tomography in The Diagnosis of Subcapsular and Perirenal HematomaGordana PuzovicNo ratings yet
- Primary Tracheal Tumors: Review of 37 CasesDocument4 pagesPrimary Tracheal Tumors: Review of 37 CasesGordana PuzovicNo ratings yet
- PIIS1556086416335158Document12 pagesPIIS1556086416335158Gordana PuzovicNo ratings yet
- Recurrent Pyogenic Cholangitis: From Imaging To InterventionDocument8 pagesRecurrent Pyogenic Cholangitis: From Imaging To InterventionGordana PuzovicNo ratings yet
- InTech-Advances Bone MetastasesDocument16 pagesInTech-Advances Bone MetastasesGordana PuzovicNo ratings yet
- Transient Hepatic Perfusion Differences (THAD/THID) - What? When? Where?Document35 pagesTransient Hepatic Perfusion Differences (THAD/THID) - What? When? Where?Gordana PuzovicNo ratings yet
- Sjogren's Syndrome, Vasculitis, and Cryoglobulinaemia Associated With (Kappa) Paraprotein With Rheumatoid ActivityDocument3 pagesSjogren's Syndrome, Vasculitis, and Cryoglobulinaemia Associated With (Kappa) Paraprotein With Rheumatoid ActivityGordana PuzovicNo ratings yet
- Prevalence of Solid Tumors in Incidentally Detected Homogeneous Renal Masses Measuring 20 HU On Portal Venous Phase CTDocument5 pagesPrevalence of Solid Tumors in Incidentally Detected Homogeneous Renal Masses Measuring 20 HU On Portal Venous Phase CTGordana PuzovicNo ratings yet
- Infection and Immunity-1971-Eudy-269.fullDocument5 pagesInfection and Immunity-1971-Eudy-269.fullGordana PuzovicNo ratings yet
- Review On Pancreatic Steatosis Detection by Imaging ModalitiesDocument10 pagesReview On Pancreatic Steatosis Detection by Imaging ModalitiesGordana PuzovicNo ratings yet
- PIIS1201971220301016Document6 pagesPIIS1201971220301016Gordana PuzovicNo ratings yet
- Role of Triple Phase Computed Tomography Findings For Evaluation of Hepatic LesionsDocument8 pagesRole of Triple Phase Computed Tomography Findings For Evaluation of Hepatic LesionsGordana PuzovicNo ratings yet
- Spontaneous Ruptured Intercostal and Lumber Artery in Type 1 NeurofibromatosisDocument15 pagesSpontaneous Ruptured Intercostal and Lumber Artery in Type 1 NeurofibromatosisGordana PuzovicNo ratings yet
- Intercostal Artery Injury Manifested by A Sentinel Pleural ClotDocument2 pagesIntercostal Artery Injury Manifested by A Sentinel Pleural ClotGordana PuzovicNo ratings yet
- 01 Cir 9 1 1Document16 pages01 Cir 9 1 1Gordana PuzovicNo ratings yet
- Early Onset Pneumonia Following Pulmonary Contusion: The Case of Stonewall JacksonDocument3 pagesEarly Onset Pneumonia Following Pulmonary Contusion: The Case of Stonewall JacksonGordana PuzovicNo ratings yet
- Focal Confluent Fibrosis in Cirrhotic Liver: Natural History Studied With Serial CTDocument7 pagesFocal Confluent Fibrosis in Cirrhotic Liver: Natural History Studied With Serial CTGordana PuzovicNo ratings yet
- Primary Aortoduodenal Fistula Caused by Aortitis: SalmonellaDocument4 pagesPrimary Aortoduodenal Fistula Caused by Aortitis: SalmonellaGordana PuzovicNo ratings yet
- Microbial and Heavy Metal Contamination in Five Brands of Kohl Used in Sulaimani CityDocument9 pagesMicrobial and Heavy Metal Contamination in Five Brands of Kohl Used in Sulaimani Citymariahernandez unefaNo ratings yet
- Application For Membership of Swimming Pool For Summer Break 2022Document2 pagesApplication For Membership of Swimming Pool For Summer Break 2022Rohit SinghNo ratings yet
- Module 1 - Community and Environmental Health ProblemsDocument4 pagesModule 1 - Community and Environmental Health ProblemsKielene PalosNo ratings yet
- Student Consent FormDocument2 pagesStudent Consent Formvmin31100No ratings yet
- Radiation ProtectionDocument20 pagesRadiation ProtectionsastoNo ratings yet
- Veterinary Medicines Injections Liquid Drench From Factory PakistanDocument17 pagesVeterinary Medicines Injections Liquid Drench From Factory PakistanSyed Ali GilaniNo ratings yet
- Assay Summary: ADVIA Centaur CPDocument14 pagesAssay Summary: ADVIA Centaur CPGuneyden GuneydenNo ratings yet
- Premature Ovarian InsufficiencyDocument23 pagesPremature Ovarian Insufficiencydr.rinanovitriani94No ratings yet
- Brochure HyBase 6100 2010 PDFDocument6 pagesBrochure HyBase 6100 2010 PDFYun EdhiharsoNo ratings yet
- Miracle Hospital Study GuideDocument31 pagesMiracle Hospital Study GuideMel ClancyNo ratings yet
- Tyler PerryDocument31 pagesTyler PerryTHR100% (4)
- Health Assessment SKILLS NOTESDocument9 pagesHealth Assessment SKILLS NOTESPauline AñesNo ratings yet
- Wisconsin Card Sorting Test (WCST)Document3 pagesWisconsin Card Sorting Test (WCST)Lauti GomezNo ratings yet
- 66294-En B - Cyclo g6 OpmanualDocument46 pages66294-En B - Cyclo g6 OpmanualRenato CastroNo ratings yet
- Nurs 451 Qualitative Critique Paper Moeandjoe 1Document7 pagesNurs 451 Qualitative Critique Paper Moeandjoe 1api-338133673No ratings yet
- (LPP) DOH Presentation On NDVPDocument19 pages(LPP) DOH Presentation On NDVPciryajamNo ratings yet
- Sindroma Dalam Psikiatri: Yuliana Ratna WatiDocument23 pagesSindroma Dalam Psikiatri: Yuliana Ratna WatiAyi Abdul BasithNo ratings yet
- Tooth Wear Against Ceramic Crowns in Posterior RegDocument9 pagesTooth Wear Against Ceramic Crowns in Posterior RegPremshith CpNo ratings yet
- Algebra 2 ProjectMag - Exp. GrowthDocument6 pagesAlgebra 2 ProjectMag - Exp. GrowthKelsey NuñezNo ratings yet
- Parvind Kumar Agarwal, MBA SEC A, Roll 62Document15 pagesParvind Kumar Agarwal, MBA SEC A, Roll 62Parvind AgarwalNo ratings yet
- Physical Fitness Test: Name: SectionDocument65 pagesPhysical Fitness Test: Name: SectionJerick SubadNo ratings yet
- Technical Standards and Guidelines For The Diagnosis of Biotinidase DeficiencyDocument7 pagesTechnical Standards and Guidelines For The Diagnosis of Biotinidase DeficiencySaad KhanNo ratings yet
- Rosen 2017Document20 pagesRosen 2017erni pabateNo ratings yet
- The Complete Cast Crown PreparationDocument55 pagesThe Complete Cast Crown Preparationaya ahmed mohamed saadNo ratings yet