Professional Documents
Culture Documents
Biforntal Polin1997 PDF
Biforntal Polin1997 PDF
Uploaded by
Fede MinghinelliOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Biforntal Polin1997 PDF
Biforntal Polin1997 PDF
Uploaded by
Fede MinghinelliCopyright:
Available Formats
TECH N IQ U E APPLICATIONS
Decompressive Bifrontal Craniectomy in the Treatment of
Severe Refractory Posttraumatic Cerebral Edema
Richard S. Polin, M .D ., Mark E. Shaffrey, M .D .,
Christopher A. Bogaev, M .D ., Nancy Tisdale, R .N .,
Teresa Germanson, Ph.D ., Ben Bocchicchio, M .S.,
John A. Jane, M .D ., Ph.D.
Department of Neurosurgery, U niversity of V irginia Health Sciences Center,
C harlottesville, Virginia
O B JEC T IV E : The management of malignant posttraum atic cerebral edem a rem ains a frustrating endeavor for the
neurosurgeon and the intensivist. M ortality and m orbidity rates remain high despite refinem ents in m edical and
pharm acological means of controlling elevated intracranial pressure; therefore, a com parison of m edical man
agement versus decom pressive craniectom y in the management of malignant posttraum atic cerebral edema was
undertaken.
M ET H O D S : At the University of Virginia Health Sciences Center, 35 bifrontal decom pressive craniecto m ies were
performed on patients suffering from malignant posttraum atic cerebral edem a. A control population w as formed
of patients whose data was accrued in the Traum atic Com a Data Bank. Patients who had undergone surgery were
matched with one to four control patients based on sex, age, preoperative G lasgow Com a Scale scores, and
maximum preoperative intracranial pressure (ICP).
R ESU LTS: The overall rate of good recovery and moderate disability for the patients w ho underw ent craniectomies
was 3 7 % (13 of 35 patients), w hereas the m ortality rate was 2 3 % (8 of 35 patients). Pediatric patients had a
higher rate of favorable outcom e (4 4 % , 8 of 18 patients) than did adult patients. Postoperative IC P w as lower
than preoperative ICP in patients who underwent decom pression (P = 0 .0 00 3 ). Postoperative IC P w as lower in
patients who underwent surgery than late m easurem ents of ICP in the m atched control population. A statistically
significant increased rate of favorable outcom es was seen in the patients w ho underwent surgery com pared to the
matched control patients (1 5 .4 % ) (P = 0.014). All patients who exhibited sustained IC P values above 40 torr and
those w ho underwent surgery more than 48 hours after the time of injury did poorly. Evaluation of the 20 patients
who did not fit into either of those categories revealed a 6 0 % rate of favorable outcom e and a statistical
advantage over control patients (P = 0.0001).
C O N C L U S IO N : Decom pressive bifrontal craniectom y provides a statistical advantage over m edical treatm ent of
intractable posttraumatic cerebral hypertension and should be considered in the m anagement of malignant
posttraumatic cerebral swelling. If the operation can be accom plished before the ICP value exceeds 40 torr for
a sustained period and within 48 hours of the time of injury, the potential to influence outcom e is greatest.
(Neurosurgery 4 1 :8 4 - 9 4 , 1 9 9 7 )
Keywords: Brain edema, Brain trauma, Intracranial pressure, Traumatic Coma Data Bank
hen routine measures fail to alleviate cerebral
W
outcomes for patients with severe, diffuse posttraumatic ce
swelling associated with traumatic brain injury in rebral edema remain poor. In the Traumatic Coma Data Bank
the absence of a mass lesion, management options (TCDB), patients who suffered diffuse injury characterized by
are few. The patients will most often either die or survive in compressed cisterns, less than 5 mm of midline shift, and no ■
an extremely disabled state. Despite our advances in under mass lesion larger than 25 cc (Diffuse Injury III) demonstrated
standing, monitoring, and treating cerebral hypertension, the only a 16.4% incidence of good outcome or moderate disabil- 1
Neurosurgery, Vol. 41, No. 1, july 1997
D eco m p ressive C ra n iecto m y in Traum a
ity at hospital discharge (17) (Table 1). Furthermore, the mor value below 20 torr and is typically administered initially in a
tality rate in this group was 34%. The most powerful predic 1 g/kg of body weight bolus and then smaller doses as
tors of mortality in these patients were the highest intracranial required to maintain an ICP value below 20 torr. In this series,
pressure (ICP) values w ithin 72 hours of injury and post hypothermia was not used and only one patient received
resuscitation pupillary examination. preoperative barbiturate therapy.
Most strategies for managing these patients rely on reduc
ing primary damage from the ischemic insult (hypothermia, O perative indications
free-radical scavengers), lowering ICP to minimize collateral
A bifrontal decompressive craniectomy was performed
damage (hyperventilation, head elevation, ventriculostomy),
when ICP remained elevated despite the aforementioned
or both (potentially mannitol, barbiturates). Because ICP ele
therapies and when a CT scan demonstrated diffuse edema
vation is a major predictor of mortality in these patients, it
without a mass lesion. In rare instances, the operation was
seems logical that maximum effort toward preventing intra
performed late in a patient's course after maximum cerebral
cranial hypertension is warranted. At the University of Virginia
swelling had subsided, but the clinical examination remained
Health Sciences Center, 35 bifrontal craniectomies for the treat
poor as a heroic measure or immediately at presentation
ment of severe refractory posttraumatic cerebral edema have
based on a CT scan appearance and clinical syndrome sug
been performed. It was hypothesized that bifrontal craniecto
gesting imminent herniation (three patients). Each of five
mies, with a slight modification from the technique presented by
patients underwent a decompressive craniectomy combined
Kjellberg and Prieto (16), would positively influence both sur
with resection of a large mass lesion, but these patients were
vival and the overall outcomes for these patients. Although
excluded from further analysis.
many authors have anecdotally reported limited degrees of
success with this approach, there has been no case-controlled
examination of the procedure. By m atching patients who Patient selection
underwent surgery in a case-controlled manner with patients Between January 1984 and September 1993, 35 patients
with the same intracranial diagnoses but no surgical interven underwent bifrontal decompressive craniectomies primarily
tion from the TCDB, we determined whether surgery impacted for malignant cerebral edema as indicated by elevated ICP or
the rate of favorable outcome in this patient population. a typical appearance on CT scans of loss of the normal gyral
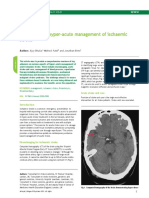
pattern and compression of the basal cisterns (Fig. 1 ,A and B).
An additional five patients underwent bifrontal craniectomies
PATIENTS AND M ETH O D S
concurrent with evacuation of traumatic mass lesions. These
Standard clinical management five were excluded from the analysis. Also excluded was a
patient who presented at the emergency room with a GCS
All patients with presumed head injuries at the University
of Virginia Hospital are treated by the neurosurgical service.
Standard management includes completion of a computed
tomographic (CT) scan as rapidly as possible at presentation
to the emergency room and placement of an ICP monitor in all
patients with a Glasgow Coma Scale (GCS) score of 10 or less
or in individuals whose examinations are blunted by neuro
muscular blockade or heavy sedation. Standard therapy for
elevated ICP includes mild hyperventilation to a P C 0 2 of 30
to 35 torr and elevation of the head of the bed. Mannitol is
administered when these measures fail to maintain an ICP
TABLE 1. Glasgow Outcom e Scores of Patients with Diffuse
-1' Injury IIU
GOS No. of Patients (%)
FIG U R E 1. CT scans obtained at admission of a 17-year-old
Good recovery 5 (3.3) boy with a G C S score of 5T (A) and a 30-year-old man with
Moderate disability 20 (13.1) a G C S score of 6T (B ) after motor vehicle accidents. There is
Severe disability 41 (26.8) evidence of diffuse cerebral edema demonstrated by poor
Vegetative 35 (22.9) grey-white matter differentiation, loss of the normal gyral
Dead 52 (34.0) pattern, and compression of the basal cisterns in the absence
Data obtained from the Traumatic Coma Data Bank. Diffuse Injury of lesions causing significant mass effect. There is some trau
ls defined as compression of cisterns. GOS, Glasgow outcome score. matic subarachnoid hemorrhage in the first patient (A).
N eu rosurgery, Vol. 41, No. 1, July 1997 85
86 Polin et al.
score of 2T after an automobile accident and who displayed
bilaterally fixed and dilated pupils. The patient was taken
emergently to the operating room, where the typical removal
of bone (as described below) was performed. When opening F IG U R E 2. Illustration of the
the dura, the operating surgeons observed evidence of an wide bifrontal craniotomy,
absence of cortical blood flow bilaterally and the procedure fishmouth dural incisions,
was terminated before completion. and dural expansion graft
Of 26 patients in whom vital signs were reviewed, preop that characterize the
erative systemic hypotension (systolic blood pressure less bifrontal craniectomy.
than 100 mm Hg) was observed in six patients. In all of the
patients, the neurological injury was the major source of
morbidity and mortality. Only one patient experienced an
extended hospital stay because of systemic illness; this patient
developed posttraumatic pancreatitis, which necessitated pro Data collection
longed hospitalization for hyperalimentation.
For the 92 control patients, limited data on systemic com Patient flow sheets were retrospectively reviewed to deter
plications were available from the TCDB. Seven patients suf mine the presenting GCS scores and results of the neurolog
fered cardiac arrests, and 31 were noted to be hypotensive at ical examinations. Intubated patients were assigned an im
some point. Shock was noted to be life-threatening in 14 plied verbal score of 1. Hourly ICP values, systolic pressure,
individuals. Only five patients were noted to have systemic and mean blood pressure, both pre- and postoperatively,
(gastrointestinal, hepatosplenic, renal, or peripheral vascular) were recorded, as were therapeutic maneuvers designed to
injury that was life-threatening. Mannitol or furosamide was reduce ICP. A Glasgow outcome score (GOS) was recorded as
administered in the early evaluation and treatment or the the outcome at the time of hospital discharge, which included
1st day in the intensive care unit for 45 of 86 patients (52%), acute inpatient care on the rehabilitation service at the Health
and barbiturates were administered to an additional three Science Center when applicable.
Patients from the TCDB with the diagnosis of diffuse injury
patients.
without significant mass lesion or midline shift were eligible
as control group patients. Anyone with a surgical decompres
O perative technique sion or a penetrating injury was excluded. Patients undergo
ing surgery and control patients were matched by sex, age,
Decompressive bifrontal craniectomies were performed and best presenting GCS score. The highest sustained preop
with the patients in the supine position with a mild degree of erative ICP values of the patients undergoing decompression
reverse Trendelenburg positioning. A bicoronal skin incision were matched with the maximum sustained ICP values of the
was made, and the temporalis muscles were reflected inferi- control patients within the first 48 hours of presentation. This
orly. Bitemporal burr holes were created, and bilateral large factor was not used for patients who underwent decompres
subtemporal decompressions were performed. Subsequently, sion emergently without ICP monitoring. In m atching control
a large bifrontal craniectomy was created, extending posteri patients to patients undergoing surgery, sex was always iden
orly into the parietal bones approximately 3 to 5 cm posterior tical and age could vary by 1 year for children 10 years of age
to the coronal sutures. Burr holes were located at either side of and younger, by 2 years for patients from 11 to 18 years of age,
the sagittal sinus at the posterior extent, bilaterally at the and up to 4 years for older patients. The admission GCS
keyhole, and bilaterally at the root of the zygoma. The final scores of the patients were also maintained within 1 point of
bone cut was made anteriorly over the sagittal sinus after those of the control patients. Maximum preoperative ICF
stripping the dura from the underlying sinus. After bleeding values were then used as a final discriminator. Each matched
was controlled, bilateral fishmouth dural incisions were control patient was included only once, and only patients who
made, extending medially to the sagittal sinus. The sagittal had never undergone evacuation of a mass lesion were eligi
sinus was then ligated and divided anteriorly between two ble as matches. The control patients were chosen by two
2-0 Nurolon sutures. This allowed a further vector of anterior blinded observers working independently and then pooling
expansion. After hemostasis was obtained and both hemi responses to include patients selected by both. The observers
spheres were inspected for subdural hematoma, dural expan were blinded to patient names and outcomes. A total of 92
sion grafts were affixed in a watertight fashion to the dural control patients were accrued for the 35 patients undergoing
openings so that all pressure was released from the underly surgery (2.63 control patients/patient undergoing surgery)
ing brain (Fig. 2). An ICP monitor was placed before closing The demographic data of the patients undergoing decompres
on the nondominant hemisphere posterior to the bone defect. sion and the TCDB control patients are listed in Table 2.
Postoperatively, head elevation and mild hypocapnia were The highest sustained ICP values during the 24 hours after
maintained but more aggressive measures for ICP control surgery were recorded for the group who underwent decom
were not necessary. Typically, the bone flap was replaced pression. As a comparison, the maximum sustained hourly
between 3 and 6 months after the original surgery. The flap ICP values of the control patients that were obtained 48 to 72
was maintained in a wet gauze at - 4 ° C until reinsertion. hours after injury were recorded. The mean 48- to 72-hour ICF
Neurosurgery, Vol. 41, No. 7, July 1997
D ecom p ressive C ra n iecto m y in Traum a 87
TABLE 2. Comparison of Baseline Characteristics of Patients Kjellberg and Prieto (16). The average patient age was 18.7
Undergoing Decompressive Craniectom ies versus those of years. Thirty-one of the 35 patients had suffered motor vehicle
the Traumatic Coma Data Bank Control Group accidents, whereas the remainder were victims of assault. In
Craniectomy Group Control Group no patient was there a non-neurosurgical injury requiring
thoracotomy or laparotomy. The admission GCS scores fol
Age'1 18.7 ± 12.6 19.1 ± 9.42 lowed a bimodal distribution, with peaks at GCS scores of 4
Admission G C S 5.6 2 ± 2.11 5.6 8 ± 1.65 (12 patients) and 7 (9 patients) (Fig. 3). The mean GCS score at
score admission was 5.62. Three patients who presented with a GCS
Maximum IC P ‘ 3 4 .9 torr ± 14.9 33.2 torr ± 1 5.6 score higher than 7 had all deteriorated substantially at the
'Age, two-tailed t test, P = 0.784. time of surgery. No patient had a GCS score higher than 7 at
'’ GCS, Glasgow Coma Scale, two-tailed t test, P = 0.781. the time of surgery. The maximum sustained preoperative
c ICP, intracranial pressure, two-tailed t test, P = 0.346. ICP values were greater than 20 torr in all except two patients.
The distribution of preoperative ICP values is provided in
values were averaged within each control group before data Figure 4.
analysis. A comparison was made between the patients who had
A GOS of good recovery or moderate disability was con- undergone a decompressive craniectomy and their matched
°R sidered to be a favorable outcome. All other outcomes were controls. The average age of the surgical series was 18.7 years,
111 considered to be unfavorable. compared to 19.1 for the weighted controls. The average
•sun preoperative GCS score was 5.62 for the patients undergoing
ei Statistical analysis surgery and 5.68 for the control patients. The average m axi
ed' mum ICP value before surgery of patients undergoing crani
e(j Statistical analysis was performed by comparing the group
ectomies was 34.9 torr. The average maximum ICP value
Ui), of 35 patients undergoing surgery to a weighted composite of
during the first 48 hours after hospital admission was 33.2 torr
eal 35 control patients. The controls for each patient were aver
in the weighted controls.
aged and reported as a single entity to avoid patients with
njj, more matched controls from dominating the analysis. Age,
jgj^ GCS score, and maximum ICP value were treated as contin- O perative and postoperative com plications
pr uous variables. For instance, the composite control for a pa- Operative complications occurred in six patients. One pa
er tient with three controls (ages 14, 15, and 16 yr; GCS scores of tient developed a frontal lobe hematoma, which necessitated
. 4,5, and 5; maximum ICP values of 31, 33, and 34 torr) would evacuation. Two patients developed skim subdural hemato
re be a 15-year-old with a GCS score of 4.67 and an ICP value of mas that were not considered symptomatic. One patient de
>SSM 32.67 torr. Pediatric patients were defined as patients who veloped cerebrospinal fluid otorrhea, which resolved with
0PI were 17 years of age or younger. To compare the array of ICP lumbar drainage that was most likely related to traumatic
p values of the decompression group before and after surgery cranial base injury. One individual developed meningitis that
rt and to compare postoperative ICP values of this group to the resolved with antibiotics. The sixth complication, a case of
Ij 48- to 72-hour ICP values of the control group, a Student's t diabetes insipidus, was most likely related to traumatic dien
jjt test and a Kruskal-Wallis analysis were performed. cephalic injury.
A conditional logistic regression analysis was performed Ten patients developed shunt-dependent hydrocephalus
ifa cornparing the 92 control patients with the 35 patients under- during their postoperative course; another two suffered bone
(v going surgery to determine whether an influence on favorable flap resorption after cranioplasty, which necessitated acrylic
pint outcome was present independent of the prognostic factors on cranioplasty. One patient, despite lower ICP, had late dilation
3 which patient matching was based. The statistical method of of his right pupil; a subsequent CT scan showed progression
tche us'n§ a multiple control patient to patient undergoing surgery of a small right temporal lobe contusion. This patient under-
swk strategy was based on the analysis presented by Breslow and
elif ^ conditional logistic regression for matched sets.
, According to the authors, a 4:1 control to subject ratio is
X,lir °Pbmal, although any ratio between 1:1 and 4:1 is feasible. A
ojve A2was generated. A P value of less than or equal to 0.05
p was considered statistically significant. For univariate analy-
•goif S*S/ ^ yalues were generated with Fisher's exact test substi-
geP tutec* when at least one cell in the comparison group had a
ipre Va*ue ^ or smaller.
\
s aft RESULTS
3 4 5 6 7 8 >8
ecof n
l0ur emo8raphics and baseline characteristics G C S S co re
Ito Twenty-four male and 11 female patients underwent the FIG U RE 3. Postresuscitation G C S scores of patients undergo
jr I1 operation that was modified from the method presented by ing decompressive craniectomies.
N eu rosurg ery, Vol. 41, No. 1, luly 1997
88 Polin et al.
TABLE 3. Discharge Glasgow Outcom e Scores of Patients
Undergoing Decompressive Craniectom ies versus 3-Month
Glasgow Outcom e Scores of Traum atic Coma Data
Bank Control
No. of Patients No. of TCDB
GOS Undergoing Control
Craniectomies (%) Patients (%)
Good recovery 4 (11.4) 7 (7.6)
M oderate disability 9 (25.7) 8 (8.8)
Severe disability 11 (31.4) 32 (35.2)
M a x im u m S u sta in e d P re -o p e ra tiv e ICP (torr) Vegetative 3 (8.6) 17 (18.7)
FIG URE 4. Maximum sustained preoperative ICP values of Dead 8 (22.9) 28 (30.8)
patients who underwent decompressive craniectomies. a G O S , Glasgow outcome score; T C D B , Traum atic Com a Data Bank,
went a small right-sided temporal lobectomy several days
after the bifrontal craniectomy. no favorable outcomes in seven patients who were operated
on after 48 hours (P = 0.022). Most patients were discharged
Control of intracranial pressure to either a rehabilitation institute, another acute care hospital
or home. Only 3 of the 27 survivors (11%) were discharged to
One goal of the analysis was to demonstrate the effect of
a chronic care facility.
decompressive craniectomy on ICP. Preoperative ICP values
Several factors were identified retrospectively as being pos
were similar between the two groups (34.9 torr in the patients
sible discriminators of favorable and unfavorable outcomes
undergoing craniectomies versus 33.2 torr in the matched
Patients were assessed for the presence of preoperative co
control patients) (highest sustained ICP value within the first
agulopathy to analyze the effect of prolonged coagulation
48 h of presentation). The maximum sustained preoperative
parameters on outcome. Eighteen patients showed elevation
ICP value of each patient was compared with the maximum
of either the prothrombin time or the activated partial throm
sustained ICP value obtained during the 24 hours after sur
boplastin time before surgery, and 17 had normal studies. The
gery for the 26 patients for whom both values were available.
difference in the rate of favorable outcome (33 versus 41%)
The mean ICP value was reduced from 32.1 torr preopera-
and mortality (28 versus 18%) between the two groups was
tively to 21.3 torr postoperatively. A paired t test revealed a
not significant.
statistically significant change in ICP in the patients who
However, patients who attained ICP values greater than 40
underwent decompression after surgery (P = 0.0001).
torr for a sustained period not associated with agitation or
The postoperative ICP values of the patients who under
went decompression were compared in a similar fashion to poor pain control and those patients not operated on within
the maximum ICP values of the weighted control patients the first 48 hours of presentation to the em ergency room had
obtained from 48 to 72 hours after injury. Twenty-nine pa poor outcomes. Only 1 of 15 (6.7%) patients meeting these two
tients had postoperative ICP measured. The mean postoper criteria had a favorable outcome, compared to 12 of 20 (60%)
ative ICP value of these patients was 21.6 torr, compared to favorable outcomes if neither criteria was met (P — 0.0004)
29.4 torr obtained between 48 and 72 hours after injury for the Each of eight patients had an ICP value greater than 40 ton
control patients. The paired t test again demonstrated a sta whereas seven had surgery delayed past the 2nd day.
tistically significant reduction in ICP (P = 0.026). Pediatric patients had better results with surgery than did
adult patients, with favorable outcomes in 8 of 18 (44%)
pediatric patients, compared to 5 of 17 (29%) adult patients
O utcom e
For the pediatric subset, the rate of favorable outcome was
Patient outcome was assessed at discharge from the Uni statistically significantly higher than for their matched con
versity of Virginia Health Sciences Center. The average hos trols (P = 0.025).
pital time, excluding fatal cases, was 63.22 ± 46.1 days (range, In a conditional logistic regression analysis comparing all
10-220 d). Four patients were discharged with good recover 92 control patients to the patients undergoing craniectomies,a
ies (return to school or work), 9 had moderate disabilities, 11 significant influence of the operation on favorable outcome
showed severe disabilities, 3 were vegetative, and 8 died was seen (Wald y2 = 6.097, P = 0.014). Medical management
before discharge (Table 3). The overall rate of unfavorable alone carried a 3.86 times greater risk of unfavorable outcome
outcome was 62.9%, whereas the mortality rate was 22.9%. than did decompressive craniectomy. This advantage was
The GCS score had a strong relationship to outcome. Only one maintained in the subset of patients who were operated or
patient who presented with a GCS score of 3 or 4 had a within 48 hours and who did not have maximum ICP values
favorable outcome, compared to two-thirds of patients pre that exceeded 40 torr (Table 4). The pediatric patients had
senting with a GCS score of 6 or greater (P = 0.0003). favorable outcomes in 8 of 18 cases compared to 9 of 41
Patients undergoing surgery within 48 hours of admission control patients (P = 0.079). However, if only patients with
had favorable outcomes in 13 of 28 cases (46%), compared to the aforementioned restrictions are considered, the advantage
N eu rosurgery, Vol. 41, No. 1, July 1997
D eco m p ressive C ra n ie cto m y in Traum a 89
TABLE 4. Favorable Outcom e and Decompressive pression may allow the edematous brain an alternative to
Craniectomy: Overall Results and Subset Analyses'* transtentorial and tonsillar herniation. Theoretically, by cre
Patients ating a vector of expansion in a frontal direction, the possi
Matched bility of upward herniation, as seen in the large parietal
Undergoing
Sample Control P Valueb
craniectomies decompressive craniectomies performed by Clark et al. (5),
Patients (%)
(%) would be minimized. The procedure is simple and allows
inspection of both cerebral hemispheres for extra-axial mass
All patients 35 (37) 92 (16) 0.014 lesions and access to frontal or anterotemporal intracranial
Optimal subsetc 20 (60) 58 (18) 0.0001 pathological abnormalities.
Pediatric (age < 1 8 yr) 18 (44) 41 (22) 0.079
Pediatric and optimal 10 (80) 25 (24) 0.002
Literature review
J Favorable outcome rates w ere used for patients undergoing de
compressive craniectomies and for control patients. The concept of wide bone removal for treatm ent of intra
b Conditional logistic regression with stratification by matching. cranial hypertension has existed since the dawn of neurosur
c Surgery performed within 48 hours of injury and no sustained gery. Harvey Cushing performed a subtem poral decompres
intracranial pressure greater than 40 torr.
sion for relief of elevated ICP related to neoplastic growth as
early as 1905 (7) and later reported the application of this
over control patients is statistically significant both for all
operation to wartime trauma (8). Surgeons at many centers
patients (P = 0.001) and for pediatric patients (P = 0.002).
have performed hemicraniectomies after removal of hem i
spheric traumatic brain lesions (10, 21). In 1968, Clark et al. (5)
DISCUSSION reported two cases of posttraum atic cerebral hypertension
that they had treated using "circum ferential craniotom ies,"
Clinical studies for posttraum atic brain edema
resulting in a 100% mortality rate. Kerr (15) presented a single
As indicated by the 16.4% rate of favorable outcome (good case of an extensive bifrontal craniectomy for trauma in a
outcome or moderate disability) for patients with severe ce patient who initially showed clinical im provement but subse
rebral edema revealed by CT scans from the TCDB (Table I) quently deteriorated and died.
(16), recovery to independent activity in these patients is rare. In 1971, Kjellberg and Prieto (16) reported the results of 73
Patients with this radiographic diagnosis and intractably ele patients undergoing extensive bifrontal craniectomies and li
vated ICP were considered in this study. No single therapeu gation of the sagittal sinus for posttraumatic injury. Overall,
tic maneuver, either pharm acological or surgical, has been only 18% of the patients survived, including 11 of 50 (22%)
shown to significantly im prove the prognosis for these pa with nonpenetrating head trauma. The vast majority dem on
tients. Criticism of each new approach has centered on two strated dilated pupils and decerebrate motor response. This
points: first, one must prove that patients exposed to the craniectomy was similar to a procedure that M iyazaki (18)
treatment and who recover well would not have done so and Miyazaki et al. (19) had presented. Venes and Collins (23)
without the treatment; second, one must show that the impact published a series of 13 patients who underwent craniecto
of the intervention is not simply to take patients who would mies that were performed using the method described by
have died and allow them to live with severe disability or in Kjellberg and Prieto (16). They documented only one survivor
a vegetative state. For these reasons, any novel intervention with a favorable outcome. Da Silva et al. (9) and Pereira et al.
must be examined in a study that incorporates a carefully (20) also reported anecdotal series, documenting their expe
chosen control population. We have undertaken such an anal rience with external bifrontal decompression for trauma.
ysis of 35 patients undergoing decompressive bifrontal crani Pereira et al. (20) operated on 12 patients with refractory
ectomies (modified from the method presented by Kjellberg cerebral edema and noted excellent results in 5 (41.7%).
and Prieto [16]) at the University of Virginia Health Sciences In 1980, Gerl and Tavan (14) reported a series of 30 patients
Center between January 1984 and Septem ber 1993. undergoing extensive dual hemispheric craniectomies for
The rationale for decompression has intuitive merit. Al trauma. This series had a 70% mortality rate and a 20% rate of
though decompression does not reverse the primary brain "full restitution." There was an average 3.2-day interval be
injury associated with traumatic injury, it can ameliorate sec tween injury and operation. Gaab et al. (13) undertook a
ondary damage caused by elevation of ICP. For this reason, prospective investigation of decompressive craniectomy, ex
surgery should be undertaken within 48 hours of injury, cluding patients older than 40 years and those with mass
before the period of maximal cerebral swelling. In this study, lesions. Of 37 patients treated, 19 underwent bifrontal crani
decompression significantly reduced ICP in the patients who ectomies and 18 underwent hemicraniectomies. Unfortu
underwent surgery (P = 0.0001). Furthermore, postoperative nately, their results were not broken down by type of opera
iCP values of the patients who underwent craniectomies were tion; however, only 5 of 37 patients died and 14 of 37
lower than those of the control patients obtained 48 to 72 demonstrated "full rehabilitation." These investigators
hours after injury (P = 0.026). This time limit was chosen for stressed the need for early intervention and claimed that full
the control patients, because most patients were operated on recovery occurred in all patients with preoperative GCS
within 48 hours; therefore, 24 hours after surgery most readily scores greater than 5 if prompt surgery was performed. Fisher
compares with postinjury Day 3 in the control group. Decom and Ojemann (11) reported anecdotally favorable recoveries
N eu rosurgery, Vol. 41, No. 1, July 1997
Polin et al.
after bifrontal craniectomies for patients with malignant cere of Virginia Health Sciences Center, so that all patients \vert
bral edema after subarachnoid hemorrhage. managed with a similar standard of care. Although the med
Although these authors have introduced into the literature ical care provided may not represent the most aggressivt
a considerable volume of anecdotal experience with the bi nonsurgical treatm ent currently available, one can assumt
frontal decompressive craniectomy, the criticism of the rele that the centers involved provided a standard that is reason
vance of this work in guiding clinical decisions is apparent. able for leading neurotrauma centers.
The outcome scales provided are more simplistic and perhaps The control patients were essentially equivalent to the op
more subjective than the GOS. There is no control group erative patients in terms of age, admission GCS score, and
matched by age, sex, GCS score, or ICP to demonstrate a poten maximum ICP. Each patient in the craniectom y group was
tial beneficial effect. There is no mention of postoperative ICP. treated with hyperventilation. An attempt was made to lower
For these reasons, although improved results have been seen in ICP chemically, with mannitol or diuretics, in all patients
the most recent studies, no conclusive evidence showing a ben except those who underwent im m ediate surgery. Each pa
efit of decompressive craniectomy has been produced. tient, at admission to the intensive care unit, was experiencing
some form of sedation or paralysis. Only one patient had a
trial of barbiturate coma. None received tirilazad or any other
Laboratory investigation study drug or experimental protocol. Sim ilarly, the TCDB
Part of the controversy surrounding the application of de patients were universally treated with aggressive manage
compression for trauma stems from the mixed results of crani ment of elevated ICP. As a whole, the patients undergoing
ectomy in experimental models of head injury. Cooper et al. craniectomies patients suffered relatively isolated closed head
(6) created cold-induced lesions in dogs and observed that injuries. There was no life-threatening abdominal or chest
craniectomized animals had lower ICP but significantly trauma. In the TCDB patients, 5 of 92 had life-threatening
higher volumes of brain edema. They postulated that lower systemic complications. Severe hypotension was seen in a
interstitial pressure caused a larger fluid egress into the brain slightly higher (15 versus 14%) percentage of TCDB patients
underlying the bone defect. Gaab et al. (12) created cold- although the absolute severity of hypotension is difficult to
induced lesions in cats and observed that craniectomy alone deduce in this retrospective review. The rate of mannitol
produced more local damage than craniectomy plus removal administration (52%) in controls is slightly lower than ex
of contused brain. However, Rinaldi et al. (22) observed that pected but may represent data collection, which includes onh
in rabbits with cold-induced lesions, craniectomy lowered the first 24 hours after injury or potentially the reliance on
ICP, normalized cerebral blood flow, and caused no addi ventricular drainage rather than osmotic diuresis as a primary
tional breakdown in the blood-brain barrier as determined by method to reduce ICP. Thirty-six patients underwent ventric
Evans blue extravasation. ulostomies, including 17 who did not receive mannitol. Over
Burkert and Paver (2) showed that the size of a of craniec all, only 18 of 92 (19.6%) control patients were known not to
tomy radius needed to be two-thirds that of the head radius to have received mannitol, furosamide, barbiturate, or a ventric
create a reduction in ICP values of at least 20 to 30 torr in an ulostomy. Although we cannot exclude the critique that the
experimental model. Burkert and Plaumann (3) reported the control patients may have been treated less aggressively than
ability to reduce ICP in an experimental canine model of brain the craniectomy patients, we again em phasize that the centers
trauma and presented the results of 19 patients treated with involved in the TCDB are leaders in neurotrauma research
bilateral frontotemporal parietal craniectomies. and care is expected to have been managed according to an
aggressive standard.
Another consideration was the difference in end points for
Control population
analysis. For TCDB patients, the 6-month GOS was used
To consider decompressive craniectomy a logical proce compared to the discharge GOS for the patients who under
dure to ameliorate the effects of cerebral hypertension, one went craniectomies. The mean length of hospitalization in the
must first prove that the operation actually leads to a reduc surgical population was 62 days, excluding fatalities. There
tion of ICP in the patient group compared to the control fore, the control patients had longer average follow-up peri
group. Subsequently, one must show an improvement in ods. Because GOS tends to improve over time (4), this may
outcome compared to a matched control population. In the have introduced a bias favoring the control population. The few
absence of a prospective, randomized trial, historical controls patients in the operative population who remained hospitalized
must be used. In this study, the TCDB has been used as a to nearly the 6-month point were universally the most impaired
source of control patients for several reasons: 1) most of our survivors, none of whom gained favorable outcomes.
patients were accrued during or within 3 years of the dates of
the TCDB, negating any effect of improved management of
the head-injured patient over time between the series; 2) the Indications for operation
TCDB was meticulously constructed so that information The usefulness of any intervention for raised ICP is im
about daily ICP, GCS score, and level of therapeutic intensity paired if the delay to operation is excessive, the ICP elevation
was recorded in the master data bank; 3) the TCDB was is too intense, or the primary mechanism of impairment is
constructed from major neurosurgical centers with an institu brain stem shear and not ICP-related. As our criteria for the
tional committment to neurotrauma, including the University bifrontal craniectomy have evolved over time, there are sowt’
N eu rosurgery , Vol. 41, No. 1, July 1997
D ecom p ressive C ra n iecto m y in Traum a 91
patients early in the series for whom this procedure may have SU M M A R Y
been overly heroic. In particular, one victim of child abuse
A retrospective analysis of 35 patients undergoing de
and several pedestrians struck by motor vehicles were oper
com pressive craniectom ies via a m odified m ethod of that
ated on more than 96 hours after the time of presentation. The
presented by Kjellberg and Prieto (16) was performed. O per
outcomes in these patients were universally poor.
ative complications were few. ICP reduction achieved by
To demonstrate the efficacy of this operation within appro
performing surgery was statistically significant (P = 0.0001).
priate clinical guidelines, criteria for the procedure were cre
Thirteen patients had favorable outcom es, and 22 had un
ated. These standards dictate that surgery should be under
favorable outcom es. For the subset of patients w ho under
taken within 48 hours and that ICP elevation should not
w ent surgery w ithin 48 hours and who never sustained ICP
exceed 40 torr. This is based on the common sense principle
values greater than 40 torr, favorable outcom es w ere ob
that patients cannot recover from certain injuries and that
served in 12 of 20 patients, com pared to 18% favorable
some intervention is too late. We argue that once the ICP
outcom es in control patients. These criteria are intended to
reaches a sustained level above 40 torr, the chance for inter
screen for patients who have herniated from m alignant
vention before permanent neurological devastation has
edema before decom pression or w ho have passed the point
passed. Similarly, if the patient is not operated on promptly,
at which recovery is possible.
sustained damage from elevated ICP can preclude recovery.
Each patient was matched with one to four patients from
Most of our failures within the entire population consisted
the TCDB with a diagnosis of diffuse edema and without
of heroic attempts to help patients recover from injuries
presence of mass lesions. W eighted composites consisting of
from which they likely could not recover. Two additional
the average of the control patients were constructed for each
failures included the patients without severe ICP elevation
patient. Comparing the 35 patients with their 35 composite
whose poor neurological states were likely secondary to dif
control patients, there was essential equivalence in average
fuse axonal injury. They underwent the procedure based on
age, GCS score, and ICP. Using a conditional logistic regres
their age and their CT scans, which demonstrated diffuse
sion analysis, the patients who underwent craniectomies had
cerebral edema. It has been discovered that although aggressive
an increased incidence of favorable outcome (P = 0.014). The
craniectomy for refractory cerebral edema is superior to medical
subgroup of patients with the aforementioned favorable clin
management of elevated ICP, it is not universally successful, and
ical characteristics had significantly more favorable outcomes
our patient selection criteria have changed accordingly. The
as well (P = 0.001). Pediatric patients showed a greater sta
presenting GCS score provides a relative contraindication. We
tistical advantage over their matched control pediatric pa
j, do not recommend operating on patients with a GCS score of 3,
tients (P = 0.079) than did adult patients. Pediatric patients
v, but patients otherwise meeting the criteria for surgery with GCS
with favorable clinical characteristics had a statistically signif
,t scores of 4 or 5 should be considered.
icant advantage over control patients (P = 0.002).
ft The malignant edema predicted in several experimental
The role of decompressive craniectomy in brain trauma
t models was not observed. This may relate to the inherent
remains controversial. Although retrospective studies cannot
b difference between a cold-induced and a traumatic brain le-
substitute for clinical trials, the careful construction of a con
itf sion. Most patients have a small area of encephalomalacia in
trol population that matches the patients in terms of sex, age,
ait noneloquent cortex adjacent to the frontal pole (Fig. 5).
ICP, and GCS score allows a valid matched analysis. Because
3i
of the limited number of centers currently performing this type
FIG U R E 5. CT scan obtained of operation, no prospective trial has been possible. Potentially,
1 month after injury of the the advantage shown in this study and the experience with
sa
d(
same patient as in Figure 1B. patient selection may help guide the formulation of prospective
id The patient presented with a studies. This series of 35 patients who underwent decompressive
iei G C S score of 6T (E2 M4VT) craniectomies for diffuse malignant brain edema showed a clear
pa and an ICP value of 30 torr, advantage of surgery over medical therapy alone. Using the
despite hyperventilation and criteria for intervention, which we have developed over time, the
ini
'fi mannitol therapy. He was potential clinical benefit is enhanced.
taken to the operating room,
IK
tin
where a decompressive
bifrontal craniectomy was
performed. The patient was A CKN O W LED G M EN TS
discharged to rehabilitation We thank Lou Pobereskin and Elizabeth Fisher for technical
after 43 days in the hospital following commands and and editorial assistance.
verbalizing, able to eat and walk without assistance, but still
confused (moderate disability). The frontal lobes are Received, April 12, 1996.
it*
sorrounded by small fluid collections without significant Accepted, February 5, 1997.
nt
mass effect. There is a small area of encephalomalacia at the Rep rin t requests: Richard S. Polin M .D., University of Virginia
rd
medial tip of the right frontal lobe. Health Sciences Center, Box 212, Charlottesville, VA 22908.
;of
N eu rosurgery, Vol. 41, No. 1, July 1997
92 Polin et al.
20. Pereira WC, Neves VJ, Rodrigues Y: Bifrontal decompressive cram
REFEREN CES
otomv as the treatment for severe cerebral edema [in Portuguese
1. Breslow NE, Day NE: Conditional logistic regression for matched Arq N europsiquiatr 35:99-111, 1977.
sets, in Davis W (ed): Statistical Methods in Cancer Research: The 21. Ransohoff J, Benjamin MV, Gage EL Jr, Epstein F: 1 lemicranie,
Analysis o f Case-controlled Studies. Lyon, International Agency for tomy in the m anagement of acute subdural hematoma. J Neuro
Research on Cancer, 1980, vol 1, pp 248-279. surg 34:70-76, 1971.
2. Burkert W, Paver HD: Decompressive trepanation in therapy 22. Rinaldi A, Mangiola A, Anile C, Maira G, Amante P, FerraresiA
refractory brain edema Iin German]. Zentralbl N eurochir 49:318- Hemodynam ic effects of decom pressive craniectomy in cold
323, 1988. induced brain oedema. Acta N eurochir Suppl (W ien) 51:394-39f
3. Burkert W, Plaumann H: The value of large pressure-relieving
1990.
trepanation in treatment of refractory brain edema. Animal ex 23. Venes JL, Collins WF: Bifrontal decom pressive craniectomy in th
periment studies, initial clinical results (in German]. Zentralbl management of head trauma. J N eurosurg 42:429^133, 1975.
Neurochir 50:106-108, 1989.
4. Choi SC, Barnes TY, Bullock R, Germanson TA, Marmarou A,
Young HF: Temporal profile of outcome in severe head injury. CO M M EN TS
J Neurosurg 81:169-173, 1994.
Polin et al. report their sizable experience in the use of decom
5. Clark K, Nash TM, Hutchison GC: The failure of circumferential
craniotomy in acute traumatic cerebral swelling. J Neurosurg
pressive bifrontal craniectomy for the treatment of posttraumati
29:367-371, 1968. refractory intracranial hypertension. In this carefully selectee
6 Cooper PR, Hagler H, Clark WK, Barnett P: Enhancement of series, the results are impressive and, along with the report-
experimental cerebral edema after decompressive craniectomy: from Gaab et al. (1, 2) and other European groups, should leac
Implications for the management of severe head injuries. N euro to a reevaluation of this procedure in carefully selected patient
surgery 4:296-300, 1979. It is important to em phasize that the patient population wa
7. Cushing H: The establishment of cerebral hernia as a decompres young and that the medical therapy that failed would be con
sive measure for inaccessible brain tumor: With the description of
sidered by many to be less aggressive than that performed in
intramuscular methods of making the bone defect in temporal
many head injurv centers. For example, ventricular drainage in
and occipital regions. Surg Gynecol O bstet 1:297-314, 1905.
general was not used and only one patient received high dose-
8. Cushing H: Subtemporal decompressive operations for the intra
cranial complications associated with bursting fractures of the of hypnotic psychotherapeutic drugs. Because of the availabilih
skull. Ann Surg 47:641-644, 1908. of newer agents, such as propofol, it is likely that some of these
9. Da Silva JA, Da Silva CE, Sousa MB: Decompression craniot patients could have been treated for intracranial hypertension
omy in acute brain edema: Apropos of 30 operated cases [in with nonsurgical therapy. Nevertheless, there is a group of pa
Portuguese]. Arq N europsiquiatr 34:232-240, 1976. tients, often young, for whom control of intracranial pressure I
10. Delashaw JB, Broaddus WC, Kassell NF, Haley EC, Pendleton (ICP) is very difficult despite the most aggressive medical treat
GA, Vollmer DG, Maggio WW, Grady MS: Treatment of right ment and for whom surgery may be life-saving. In theory, a
hemispheric cerebral infarction by hemicraniectomy. Stroke 21:
randomized clinical trial is highly desirable, but the logistic
874-881, 1990.
render such an endeavor almost impossible to accomplish. Thu I
11. Fisher CM, Ojemann RG: Bilateral decompressive craniectomy for
worsening coma in acute subarachnoid hemorrhage: Observa the case control method that was used here, although not perfect
tions in support of the procedure. Surg Neurol 41:65-74, 1994. at least offers the reader a reasonable group with which to
12. Gaab M, Knoblich OE, Fuhrmeister U, Pflughaupt KW, Dietrich compare medical therapy with bifrontal craniectomy.
K: Comparison of the effects of surgical decompression and re One rather unusual observation in the study is the high
section of local edema in the therapy of experimental brain trau frequency of shunt-dependent hydrocephalus. In most largt
ma: Investigation of ICP, EEG and cerebral metabolism in cats. series of severe head injury, specifically in patients with diffuse
Childs Brain 5:484-498, 1979. brain swelling, shunt-dependent hydrocephalus develops in
13. Gaab MR, Rittierodt M, Lorenz M, Heissler HE: Traumatic brain
fewer than 10% of the patients. In this study, it developed in
swelling and operative decompression: A prospective investiga
more than one-third of the survivors (10 of 27 patients). Th
tion. Acta Neurochir Suppl (Wien) 51:326-328, 1990.
mechanism by which this developed is completely unclear, bu
14. Gerl A, Tavan S: Bilateral craniectomy in the treatment of severe
traumatic brain edema [in German], Zentralbl N eurochir 41:125- this observation deserves further investigation.
138, 1980. As have other authors, these authors argue that for decom
15. Kerr FW: Radical decompression and dural grafting in severe pressive craniectomy to be effective, it must be performed earl
cerebral edema. M ayo Clin Proc 43:852-864, 1968. and before the situation is irreversible. What we need in th
16. Kjellberg RN, Prieto A Jr: Bifrontal decompressive craniotomy for future is a better definition and stringent criteria for regarding
massive cerebral edema. J Neurosurg 34:488-493, 1971. medical therapy as having failed, so that surgery candidates car
17. Marshall LF, Marshall SB, Klauber MR, van Buham, Clark M,
be more accurately targeted. I think that if the ICP value in'
Eisenberg HM, Jane JA, Luerssen TG, Marmarou A, Foulkes MA:
patient with diffuse brain swelling remains greater than 25 tor
A new classification of head injury based on computerized to
in the first 48 hours despite vigorous therapy, including th
mography. J Neurosurg 75[Suppl 1]: 14-20, 1991.
18. Miyazaki Y : Bifrontal external decompression for traumatic brain administration of hypnotic psychotherapeutic drugs, and th
edema. Jpn J Surg 1:83-92, 1971. cerebral perfusion pressure cannot be kept above 70 mm Hf
19. Miyazaki Y, Hirai H, Hachisu Y, Takada I: Bifrontal external decompressive craniectomy should be considered.
decompression for traumatic brain edema [in Japanese]. Shujutsu Law rence F. Marshal
20:845-852, 1966.
San Diego, California
N eurosurgery, Vol. 41, No. 1, July 1997
D eco m p ressive C ra n iecto m y in Traum a 93
more strongly supported by a prospective trial of craniectom y
1. Gaab M, Knoblich OE, Fuhrm eister U, Pflughaupt KW, Dietrich versus more aggressive medical modalities, such as hypother
K: Comparison of the effects of surgical decom pression and re mia or barbiturates, with the potential for crossover to crani
section of local edema in the therapy of experim ental brain trau ectomy if medical management fails.
ma: Investigation of ICP, EEG and cerebral metabolism in cats.
Fred G. Barker II
Childs Brain 5:484-498, 1979.
R obert G. O jem ann
2. Gaab MR, Rittierodt M, Lorenz M, Heissler HE: Traum atic brain
swelling and operative decom pression: A prospective investiga
Boston, M assachusetts
tion. Acta Neurochir Suppl (W ien) 51:326-328, 1990.
1. Bailar JC III, Louis TA, Lavori PW, Polansky M: Studies without
Sometimes, simple solutions work best. Every neurosurgeon internal controls, in Bailar JC III, M osteller F (eds): M edical Uses o f
knows that severe closed head injury often results in increased Statistics. Boston, NEJM Books, 1992, ed 2, pp 105-123.
ICP and a bad outcome. The intervention studied here has an 2. Diehl LF, Perry DJ: A com parison of randomized concurrent
appealing rationale, to improve outcome by providing a radical control groups with matched historical control groups: Are his
decompression that decreases ICP. There seems to be little doubt torical controls valid? J C lin O ncol 4:1114-1120, 1986.
3. M iller JN , Colditz GA, M osteller F: How study design affects
that the operation was effective in reducing ICP. The authors
outcomes in com parisons of therapy: II— Surgical. Stat Med
infer a significant improvement in outcomes as well; 37% of their
8:455-466, 1989.
patients had favorable results, compared to approximately 15% 4. Sacks H, Chalm ers TC, Smith H Jr: Randomized versus historical
in a matched control group. This implies one additional favor controls for clinical trials. Am J Med 72:233-240, 1982.
able outcome for every five patients treated.
The methodology used, comparison to a matched historical In this article, Polin et al. provide reasonably convincing
control group, is a marked im provement over prior studies evidence that bifrontal decom pressive craniectomies can help
that used comparison to external, literature-based controls. improve outcomes for a selected group of patients with dif
However, there are well-known problems with historical con fuse brain swelling. This study is im portant for two reasons:
trol groups, even when carefully matched (1-4). The compa 1) it validates the use of an operation that has anecdotally and
rability of treated and control patients can be difficult to variably peppered the neurosurgical literature for many
assess because of intangible factors that defy explicit matching years, and 2) it is a good example of the use of case-controlled
strategies. For example, only three decompressive craniecto study design to look at issues that would be practically or
mies per year were performed at the study center, and the financially difficult to subject to a prospective randomized
mean age of the patients was only 18 years. This suggests that clinical trial.
this aggressive approach may have been used selectively for As do all good studies, this one raises issues that remain
younger patients. Although the controls were matched for unanswered. 1) At the University of Virginia Hospital, sub
age, other less easily identified (and nonmatchable) prognos arachnoid bolts have been preferentially used long after they
tic factors may have guided physicians in selection of patients were virtually abandoned in favor of ventriculostomies by the
for surgery, biasing the study in favor of craniectomy. In the group in Richmond that designed them. I wonder whether
absence of a prospective protocol, it is difficult to tell whether better ICP controls with ventricular drainage would have
some patients who were nominally eligible for craniectomy reduced the number of patients who would have required
according to the stated criteria did not undergo surgery be decompressive surgery. 2) Because early intervention seems
cause of physician or family choice. Another difficulty is that to optimize outcome, I wonder whether performing a decom
the control patients were not matched for postresuscitation pression and removing the bone flap at the initial operation
pupillary examination, a major prognostic factor. would be advisable for patients who seem to be at significant
The comparability of treated and control groups also de risk for brain swelling. Would the added time, potential mor
pends on the intensity of the medical management of ICP, bidity, cosmetic disfigurement, and cost (including a subse
eg., the use of barbiturates. Although there is no conclusive quent operation for replacement of the flap) be justified?
evidence that barbiturates affect outcome in postraumatic Nevertheless, this study suggests that bifrontal decompres
coma, they do have an effect on ICP. Potentially, the control sion should be considered for patients who are likely to
patients could have had more serious brain injuries than the recover (patients with GCS scores greater than 3) from severe
patients they were matched to and yet could have had similar head injuries and whose brain swelling is refractory to max
ICP values because of barbiturate treatment. This would bias the imal medical therapy.
results in favor of craniectomy. Other medical treatments for Raj K. Narayan
elevated ICP may not have been used equally in the treated and Philadelphia, Pennsylvania
control groups; the biases resulting from such imbalances cannot
be readily predicted or adjusted for post hoc. This article reviews some of the ancient and modern studies
This is a provocative and thoroughly analyzed report of a of extensive craniectomy for brain injury. Surgeons, both past
treatment that may deserve wider use, although the favorable and current, have intuitively studied the use of extensive
outcome rate was still poor (7%) for patients with a preoper craniectomy in this population of patients for whom medical
ative GCS score of 3 or 4. The general applicability of the modalities and cerebrospinal fluid drainage have produced
authors' results to a broader population of patients would be poor results. Unfortunately, the present article by Polin et al.
N eu rosurgery, Vol. 41, No. I, July 199/
94 Polin et al.
does not prove or disprove the value of craniectomy in exten erature suffer from failure to measure ICP values or to objec
sive head injury, and we are still left at the intuition stage. The tively evaluate outcomes.
case control method does not provide a definitive answer. I In this study, Polin et al. treated a very restricted group of
have several criticisms of the way these authors performed patients. They excluded patients with intracranial hematomas
the “matches." I am not convinced that comparable cases have and considered only those with elevations of ICP that re
been matched in this study, even though a serious attempt mained uncontrolled by medical therapy. O f the patients
was made. There are too many variables in these trauma studied, they observed, with one exception, that those with a
populations to be assured that comparable cases have been GCS score of 3 or 4 did not recover.
selected, and the validity of the results must therefore be Should one conclude that bifrontal craniectom y is an ap
seriously questioned. However, the article serves an impor propriate management strategy for patients with posttrau-
tant purpose, which is to encourage organized neurosurgery matic cerebral edema with GCS scores of 5 or greater and
to consider this therapeutic strategy. elevated ICP refractory to medical management who do not
have intracranial hematomas? At this time, I answer this
Charles H. Tator question with an em phatic no. Although Polin et al. used
Toronto, Ontario, Canada "control patients" from the Traumatic Coma Data Bank in an
imaginative way, this is not a substitute for a randomized
This study of the outcomes of patients treated with bifron
prospective study. The head injury literature is replete with
tal craniectomies for elevations of ICP refractory to medical
therapies that seemed promising but were not proved to be
management is another addition to the considerable literature
efficacious after more rigorous examination. It is thus impor
on the subject. Although some authorities have observed ben
tant that this study be repeated (probably in a cooperative
efits from craniectomies performed using a variety of tech
venture) in a prospective fashion with randomized controls.
niques, most have not. In a retrospective analysis examining
the effects of hemicraniectomy in a series of patients with Paul R. Cooper
acute subdural hematoma who were decerebrate or flaccid at New York, New York
the time of presentation, more than 90% of the patients had
unsatisfactory outcomes (1). The futility of craniectomy for
this group of patients contradicted the beneficial effects of this
1. Cooper PR, Rovit R, Ransohoff J: H em icraniectom y in the treat
procedure reported by some of the same authors at the same
ment of acute subdural hematoma: A re-appraisal. Surg Neurol
institution several years previously (2). The differing results 5:25-28, 1975.
of these two studies emphasizes the dangers in drawing con 2. Ransohoff J, Benjamin MV, Gage EL Jr, Epstein F: Hemicraniec
clusions from studies that are not performed prospectively tomy in the m anagement of acute subdural hematoma. J Neuro
with randomized matched controls. Other studies in the lit surg 34:70-76, 1971.
A N N O U N CEM EN T
1997 Annual Meeting
Congress of N eurological Surgeons
Earnest N. Morial Convention Center
New Orleans, Louisiana
September 27 through October 2
Mitchel S. Berger, Meeting Chairman
Mark N. Hadley, Program Chairman
Frank Culicchia, Local Arrangements Chairman
Neurosurgery, Vol. 41, No. 1, july 1997
You might also like
- NAATI CCL Urdu VocabularyDocument6 pagesNAATI CCL Urdu VocabularyCypto Expert100% (1)
- Carotid Endarterectomy: Experience in 8743 Cases.Document13 pagesCarotid Endarterectomy: Experience in 8743 Cases.Alexandre Campos Moraes AmatoNo ratings yet
- MCQ Anaesthesia QuestionsDocument4 pagesMCQ Anaesthesia Questionsapi-2629165181% (32)
- Hemorragia IntracerebralDocument9 pagesHemorragia IntracerebralEdwin Jofred Torres HuamaniNo ratings yet
- Deccompresive Brain InjuryDocument4 pagesDeccompresive Brain InjuryDharmendra WidetyaNo ratings yet
- Preliminary Findings of The Minimally-Invasive Surgery Plus Rtpa For Intracerebral Hemorrhage Evacuation (Mistie) Clinical TrialDocument5 pagesPreliminary Findings of The Minimally-Invasive Surgery Plus Rtpa For Intracerebral Hemorrhage Evacuation (Mistie) Clinical TrialOmer AhmedNo ratings yet
- Jurnal Inter CS 2Document16 pagesJurnal Inter CS 2Grace YutoNo ratings yet
- Minimally Invasive Treatment For Intracerebral Hemorrhage: E A, M.D., F. H, M.D., D W. N, M.DDocument7 pagesMinimally Invasive Treatment For Intracerebral Hemorrhage: E A, M.D., F. H, M.D., D W. N, M.DThiago SouzaNo ratings yet
- 4 - 01-03-07 - J Neurosurg 2007 - Decompressive Hemicraniectomy in Malignant MCA InfarctionDocument7 pages4 - 01-03-07 - J Neurosurg 2007 - Decompressive Hemicraniectomy in Malignant MCA InfarctionThiago Scharth MontenegroNo ratings yet
- Icp MonitoringDocument6 pagesIcp Monitoringfarhanfarwani familyNo ratings yet
- (10920684 - Neurosurgical Focus) Decompressive Hemicraniectomy For Spontaneous Intracerebral HemorrhageDocument6 pages(10920684 - Neurosurgical Focus) Decompressive Hemicraniectomy For Spontaneous Intracerebral HemorrhageSabyaNo ratings yet
- Anestesi Luar BiasaDocument51 pagesAnestesi Luar BiasarozanfikriNo ratings yet
- Acute Subdural Hematoma Morbidity, Mortality and Operative TimingDocument7 pagesAcute Subdural Hematoma Morbidity, Mortality and Operative TimingNadia BordaşNo ratings yet
- Treatment SDH ChronicDocument6 pagesTreatment SDH ChronictwindaNo ratings yet
- CC 8178Document7 pagesCC 8178Mario Fernando A LNo ratings yet
- Surveillance IchDocument6 pagesSurveillance IchGabriel Septiana CitraNo ratings yet
- New England Journal Medicine: The ofDocument12 pagesNew England Journal Medicine: The ofBill BerilNo ratings yet
- Medi 98 E18430Document2 pagesMedi 98 E18430Anonymous tG35SYROzENo ratings yet
- Trial of Decompressive Craniectomy For Traumatic Intracranial HypertensionDocument12 pagesTrial of Decompressive Craniectomy For Traumatic Intracranial HypertensionV ANo ratings yet
- ICH SurgeryDocument14 pagesICH SurgeryJunus EufrataNo ratings yet
- Persistent Posttraumatic Cerebrospinal Fluid Leakage: J A. F, M.D., M J. E, M.D., L M. Q, R.NDocument5 pagesPersistent Posttraumatic Cerebrospinal Fluid Leakage: J A. F, M.D., M J. E, M.D., L M. Q, R.NAlexanderCahyadiNo ratings yet
- Decompressive Hemicraniectomy Associated With Ultrasound-Guided Minimally Invasive Puncture and DrainageDocument11 pagesDecompressive Hemicraniectomy Associated With Ultrasound-Guided Minimally Invasive Puncture and Drainageclaudio RivasNo ratings yet
- Protocols: Destiny Ii: Decompressive Surgery For The Treatment of Malignant Infarction of The Middle Cerebral Artery IiDocument8 pagesProtocols: Destiny Ii: Decompressive Surgery For The Treatment of Malignant Infarction of The Middle Cerebral Artery Iirodolfo riosNo ratings yet
- Follow-Up Computed Tomography After Evacuation of Chronic Subdural HematomaDocument3 pagesFollow-Up Computed Tomography After Evacuation of Chronic Subdural HematomaSantiago Sanchez Jr.No ratings yet
- 215 FullDocument12 pages215 FullGusti AnisaNo ratings yet
- Turkish Neurosurg 13 - Surgical Management of TISDHDocument6 pagesTurkish Neurosurg 13 - Surgical Management of TISDHZeptAlanNo ratings yet
- Marshall1991 The Outcome of Severe Closed Head InjuryDocument9 pagesMarshall1991 The Outcome of Severe Closed Head InjuryJulieta PereyraNo ratings yet
- ICU Trial SummariesDocument31 pagesICU Trial SummariesSimon WongNo ratings yet
- Journal ReadingDocument53 pagesJournal ReadingRhadezahara PatrisaNo ratings yet
- Intracranial Pressure Management in Patients With Traumatic Brain Injury: An UpdateDocument5 pagesIntracranial Pressure Management in Patients With Traumatic Brain Injury: An UpdatedimasNo ratings yet
- Intracerebeller HemorrhageDocument9 pagesIntracerebeller HemorrhageWenny KalamiNo ratings yet
- Stich IDocument11 pagesStich IKevin Mora BañosNo ratings yet
- Chest Wall, Pneumothorax, and Hemothorax: Brain DeathDocument5 pagesChest Wall, Pneumothorax, and Hemothorax: Brain DeathMelly SyafridaNo ratings yet
- Fmed 09 799310Document4 pagesFmed 09 799310Chris Chavez CollazosNo ratings yet
- Craniectomia DescompresivaDocument51 pagesCraniectomia DescompresivaVlady78No ratings yet
- Surgical Treatment of Traumatic Bifrontal Contusion When and HowDocument37 pagesSurgical Treatment of Traumatic Bifrontal Contusion When and HowNGUYỄN HOÀNG LINHNo ratings yet
- Eriksen Et Al-2002-Clinical Cardiology PDFDocument5 pagesEriksen Et Al-2002-Clinical Cardiology PDFmedskyqqNo ratings yet
- Pan 2017Document1 pagePan 2017Arhip CojocNo ratings yet
- SNH CraniotomyDocument8 pagesSNH CraniotomyxshreNo ratings yet
- Penyaji: Ayu Iswandari Raharjo Pembimbing 1:Dr. Dr. M. Zafrullah Arifin, SP - Bs (K) Dr. Ahmad Faried, SP - Bs (K) PHDDocument14 pagesPenyaji: Ayu Iswandari Raharjo Pembimbing 1:Dr. Dr. M. Zafrullah Arifin, SP - Bs (K) Dr. Ahmad Faried, SP - Bs (K) PHDayuNo ratings yet
- Postoperative Meningitis After Spinal Surgery: A Review of 21 Cases From 20,178 PatientsDocument5 pagesPostoperative Meningitis After Spinal Surgery: A Review of 21 Cases From 20,178 PatientsntnquynhproNo ratings yet
- Management of Neoplastic Pericardial EffusionsDocument4 pagesManagement of Neoplastic Pericardial EffusionsMamamia DonchanNo ratings yet
- Surgery For Intracerebral Hemorrhage: Emerging Therapy CritiquesDocument3 pagesSurgery For Intracerebral Hemorrhage: Emerging Therapy CritiqueshafizalfarizieNo ratings yet
- J Neurosurg Article p1227Document8 pagesJ Neurosurg Article p1227jonas lopetNo ratings yet
- Imagingofacutestroke: Current StateDocument9 pagesImagingofacutestroke: Current Statealejandro echeverriNo ratings yet
- Decompressive Craniectomy: Indications and Techniques: Craniotomia Descompressiva: Indicações e TécnicasDocument6 pagesDecompressive Craniectomy: Indications and Techniques: Craniotomia Descompressiva: Indicações e Técnicasgeraldi radityaNo ratings yet
- Manual Aspiration Thrombectomy in Acute Myocardial Infarction: A Clinical ExperienceDocument9 pagesManual Aspiration Thrombectomy in Acute Myocardial Infarction: A Clinical ExperienceArdiana FirdausNo ratings yet
- Clinical-CT Correlations in TIA, RIND, and Strokes With Minimum ResiduumDocument5 pagesClinical-CT Correlations in TIA, RIND, and Strokes With Minimum ResiduumDewanggaWahyuPrajaNo ratings yet
- Strokeaha 107 485235Document12 pagesStrokeaha 107 485235rodolfo riosNo ratings yet
- Anaesthesia 1Document9 pagesAnaesthesia 1Sreekanth JangamNo ratings yet
- JofIMAB 2019 25 2p2471 2475Document5 pagesJofIMAB 2019 25 2p2471 2475afriskha bulawanNo ratings yet
- Outcome of Children With Severe Traumatic Brain Injury Who Are Treated With Decompressive CraniectomyDocument7 pagesOutcome of Children With Severe Traumatic Brain Injury Who Are Treated With Decompressive CraniectomyCarmen Juscamaita VegaNo ratings yet
- Chirurgia Hemoragiei Din Ganglionii BazaliDocument8 pagesChirurgia Hemoragiei Din Ganglionii BazaliAndreea DanielaNo ratings yet
- Left Ventricular Dysfunction Predicted by Early Troponin I Release After High-Dose ChemotherapyDocument6 pagesLeft Ventricular Dysfunction Predicted by Early Troponin I Release After High-Dose ChemotherapydeyaNo ratings yet
- 1 s20 S1878875022002145 Main - 220520 - 142507Document7 pages1 s20 S1878875022002145 Main - 220520 - 142507Angga FirmansyahNo ratings yet
- Decompressive Hemicraniectomy in Malignant Infarction of Theinternal Carotid ArteryDocument5 pagesDecompressive Hemicraniectomy in Malignant Infarction of Theinternal Carotid ArteryPrathmesh MishraNo ratings yet
- (Journal of Neurosurgery) Neuroendoscopic Approach To Intraventricular LesionsDocument10 pages(Journal of Neurosurgery) Neuroendoscopic Approach To Intraventricular LesionsAniaNo ratings yet
- Post Reteplase Evaluation PDFDocument5 pagesPost Reteplase Evaluation PDFAnonymous ysrxggk21cNo ratings yet
- Jurnal 20Document7 pagesJurnal 20Zella ZakyaNo ratings yet
- Crane Otomi ADocument11 pagesCrane Otomi ATemoc TemocNo ratings yet
- BohnstedtDocument10 pagesBohnstedtPGY 6No ratings yet
- Atlas of Coronary Intravascular Optical Coherence TomographyFrom EverandAtlas of Coronary Intravascular Optical Coherence TomographyNo ratings yet
- Jaundice: Newborn To Age 2 Months: Education GapDocument14 pagesJaundice: Newborn To Age 2 Months: Education GapRaul VillacresNo ratings yet
- Neonatal Palliative Care: Current Opinion in Pediatrics January 2017Document7 pagesNeonatal Palliative Care: Current Opinion in Pediatrics January 2017Raul VillacresNo ratings yet
- Cirugia Prenatal Del MielomeningoceleDocument7 pagesCirugia Prenatal Del MielomeningoceleRaul VillacresNo ratings yet
- Amiodarone Versus Procainamide For The Acute Treatment of Recurrent Supraventricular Tachycardia in Pediatric PatientsDocument7 pagesAmiodarone Versus Procainamide For The Acute Treatment of Recurrent Supraventricular Tachycardia in Pediatric PatientsRaul VillacresNo ratings yet
- Management of Childhood Seizure in Pediatric Emergency Department 237 PDFDocument4 pagesManagement of Childhood Seizure in Pediatric Emergency Department 237 PDFRaul VillacresNo ratings yet
- Journal of Neonatal Nursing: Katrina Diamandopoulos, Janet GreenDocument8 pagesJournal of Neonatal Nursing: Katrina Diamandopoulos, Janet GreenRaul VillacresNo ratings yet
- Necrotizing Enterocolitis Incidence, Characteristics, and Outcomes in Neonatal Down Syndrome PatientsDocument6 pagesNecrotizing Enterocolitis Incidence, Characteristics, and Outcomes in Neonatal Down Syndrome PatientsRaul VillacresNo ratings yet
- Down SyndromeDocument8 pagesDown SyndromeRaul VillacresNo ratings yet
- Paediatric Respiratory Reviews: Paris C. Papagianis, J.J. Pillow, Timothy J. MossDocument8 pagesPaediatric Respiratory Reviews: Paris C. Papagianis, J.J. Pillow, Timothy J. MossRaul VillacresNo ratings yet
- Displasia BroncopulmonarDocument12 pagesDisplasia BroncopulmonarRaul VillacresNo ratings yet
- Evaluation of Aortic Intima-Media Thickness in Newborns With Down SyndromeDocument4 pagesEvaluation of Aortic Intima-Media Thickness in Newborns With Down SyndromeRaul VillacresNo ratings yet
- Displasia BroncopulmonarDocument4 pagesDisplasia BroncopulmonarRaul VillacresNo ratings yet
- Surgical Pathology - Diseases of The Central Nervous System 401 - Dr. Baby Lynne Asuncion - October 20-28, 2014Document10 pagesSurgical Pathology - Diseases of The Central Nervous System 401 - Dr. Baby Lynne Asuncion - October 20-28, 2014atb_phNo ratings yet
- HMODocument5 pagesHMOdocaisaNo ratings yet
- Critizition of The JournalDocument20 pagesCritizition of The Journallie_bhiechanNo ratings yet
- Child Clinical Interview Form OnlineDocument24 pagesChild Clinical Interview Form OnlineMonica Bolocan100% (3)
- Trans CulturalDocument97 pagesTrans CulturalkewlangotNo ratings yet
- Antifoams ChartDocument1 pageAntifoams ChartTariq Khan OtmanzaiNo ratings yet
- MacKenzie - What Would A Good Doctor Do? Re Ections On The Ethics of MedicineDocument4 pagesMacKenzie - What Would A Good Doctor Do? Re Ections On The Ethics of MedicineCecitorNo ratings yet
- The Mexico City PrinciplesDocument26 pagesThe Mexico City PrinciplesIvan Cris SolisNo ratings yet
- DR Sagar Jawale CVDocument3 pagesDR Sagar Jawale CVSagar JawaleNo ratings yet
- Singkatan MnemonicsDocument4 pagesSingkatan MnemonicsguterizalNo ratings yet
- Official ResumeDocument2 pagesOfficial Resumeapi-268469441No ratings yet
- Vaccination RecordDocument15 pagesVaccination RecordFerdinand LucasNo ratings yet
- L4-SCBM 343-4 CBC 45-43-WJDocument43 pagesL4-SCBM 343-4 CBC 45-43-WJpond_1993No ratings yet
- Community Diagnosis Barangay Mabato: Presented ToDocument73 pagesCommunity Diagnosis Barangay Mabato: Presented ToRose AnnNo ratings yet
- Zoology ProjectDocument9 pagesZoology ProjectPorkodi ShanmugamNo ratings yet
- A Study To Evaluate The Effectiveness of Massaging of Foot On The Level of Pain Among Post-Operative Patient at Selected Hospital of BadamiDocument4 pagesA Study To Evaluate The Effectiveness of Massaging of Foot On The Level of Pain Among Post-Operative Patient at Selected Hospital of BadamiInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Pharmacology - Endocrine DrugsDocument123 pagesPharmacology - Endocrine DrugsBenjamin Joel BreboneriaNo ratings yet
- NeuroendosDocument25 pagesNeuroendosBabu Ramakrishnan100% (1)
- Association of Bjork Structural Signs of Growth Rotation and Skeletofacial MorphologyDocument4 pagesAssociation of Bjork Structural Signs of Growth Rotation and Skeletofacial MorphologyMargarida Maria LealNo ratings yet
- Community Medicine ME213 - Group 1 Research Team Advisor: Prof. Hematram YadavDocument55 pagesCommunity Medicine ME213 - Group 1 Research Team Advisor: Prof. Hematram YadavThulasi tootsieNo ratings yet
- Sample Questions Nursing ReviewDocument4 pagesSample Questions Nursing ReviewJenny TorredaNo ratings yet
- Safety Data Sheet Crodasinic Ls30: 1. Identification of The Substance/Preparation and of The Company/UndertakingDocument5 pagesSafety Data Sheet Crodasinic Ls30: 1. Identification of The Substance/Preparation and of The Company/UndertakingrafaeldelperuNo ratings yet
- 906-Article Text-2472-1-10-20221228Document9 pages906-Article Text-2472-1-10-20221228Kintan ShabillaNo ratings yet
- AnemiaDocument9 pagesAnemiaMila Canoza HerreraNo ratings yet
- Intermediate Life SupportDocument21 pagesIntermediate Life SupportWarren Da-mac RexNo ratings yet
- Miller - No Notice Mci Exercise Facilitator ChecklistDocument2 pagesMiller - No Notice Mci Exercise Facilitator Checklistapi-431563289No ratings yet
- Band and Loop Space Maintainer: Fabrication RequirementsDocument1 pageBand and Loop Space Maintainer: Fabrication RequirementsVidhi AdilNo ratings yet
- Insect Pathology and Entomopathogens PDFDocument33 pagesInsect Pathology and Entomopathogens PDFEduardo Urbano Moraga CáceresNo ratings yet