Professional Documents
Culture Documents
Ougs Wessex Branch Agm On 25 Jan 2020
Uploaded by
api-3432397080 ratings0% found this document useful (0 votes)
57 views1 pageOriginal Title
ougs-wessex-branch-agm-on-25-jan-2020
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
57 views1 pageOugs Wessex Branch Agm On 25 Jan 2020
Uploaded by
api-343239708Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
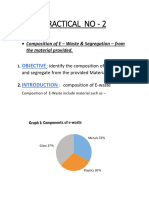
Wessex Branch Day of Lectures: Hidden Worlds 25 January 2020
Dr Laura Newsome (Camborne School of Mines): Making microbes work for us
Dr Newsome began her fascinating presentation by describing the range, abundance,
tolerance and metabolic capabilities of microbes, which include bacteria, archaea, fungi,
protozoa, algae and viruses. Archaea and bacteria are single-celled, the rest are multi-
celled – and all are extremely tiny, as small as 1μm (1/1000 mm) in diameter.
What microbes lack in size, they make up for in abundance. Bacteria alone have a
combined mass on Earth equivalent to 50 million blue whales. Archaea produce 90% of
the world's methane. And, although it wasn't discovered until 2002, the bacterium
Pelagibacter ubique turns out to be the most abundant free-living organism known.
Microbes thrive in different conditions varying from alkaline chalk to acid sulfidic Laura Newsome
rocks. They live in communities and can multiply in almost any environment where
there is free water, even deep within rocks.
The vitality of microbes is expressed by their
ability to metabolise. They "eat" by taking up
energetic electrons and "breathe" by giving up
electrons. In the sediment column, microbes
in the oxygen-rich environment at the top will
metabolise rapidly. Lower down, others will
reduce other compounds, such as manganese
IV and iron III, releasing less energy. At the
bottom, methanogenesis maintains small
Microbial metabolism
Image courtesy of Dr Laura Newsome with sediment column from
single-celled prokaryotic archaea – a meta-
Hannes Grobe [CC BY (https://creativecommons.org/licenses/by/3.0)] bolic style likened to “living on lettuce”.
By changing the oxidation state of metal ions and hence their solubility, microbes can change the
partitioning of metals between water, soil and rock. This allows mining of a target or decontamination
of a toxic site. Dr Newsome’s research concerns biomining of cobalt, decontamination of the Sellafield
nuclear site and the nature and tolerance of the microbial community in former metal and coal mines.
To make the microbes work, you have to feed them. An example of this with adverse effects for
humans is seen in Bangladesh where weathering of arsenopyrite leads to the accumulation of arsenic
(V) and iron oxyhydroxides in aquifers. Organic matter in the form of agricultural waste feeds
microbes and stimulates the reduction of iron oxyhydroxides, releasing arsenic to groundwater and
exposing the population to dangerous amounts.
Bacteria that can oxidise metals and sulfides are currently used to mine Microbial metabolism
about 15% of copper and 5% of gold globally. But an element that is can control how
increasingly in demand, for uses ranging from wind turbines to electric car metals behave in the
batteries, is cobalt. Dr Newsome has found that 64% of available cobalt can environment, and we
be extracted from laterites by sequentially increasing acidity in the presence can stimulate these
processes to
of a consortium of bacteria including laboratory-grown Geobacter
precipitate metals
sulfurreducens. Also that by increasing the levels of organic substrates, such and form minerals,
as glucose, some bacteria will reduce manganese and release the associated or to solubilise
cobalt into solution. Dr Newsome has now proposed a two-step technique metals and minerals.
using glucose and acetic acid to promote microbial extraction of cobalt.
Dr Newsome showed that these reactions are not only useful for mining metals but can also be used to
clean up metal contamination. At Sellafield in Cumbria, Serratia sp. are present in the soil and, when
fed fumarate and glycerol, precipitate uranium from groundwater. If this reaction can be encouraged
underground it could prevent leaching of uranium from contaminated areas. Aseptic sampling from
former lead mines in the Mendip Hills shows that there are prokaryotes and fungi known to be
oxidisers of manganese (II) present. Also, heavily iron-polluted mine waters in Saltburn, Yorkshire
have been treated in settlement ponds where bacterial reactions precipitate the metal and the resulting
fluid is further cleaned by passing through reed beds.
Bill Hinton and Mike Grover
Wessex Footnotes March 2020 Page 5
You might also like
- Biomineralization and BiominingDocument4 pagesBiomineralization and BiominingkanishkaNo ratings yet
- Environmental Microbiology - 5Document19 pagesEnvironmental Microbiology - 5Cathe RodriguezNo ratings yet
- The Geomicrobiology of Supergene Metal DepositsDocument7 pagesThe Geomicrobiology of Supergene Metal DepositsFranco Quispe VidalNo ratings yet
- HahahaDocument22 pagesHahahaDien NovitaNo ratings yet
- 07 Rincon-Tomasetal2016Document12 pages07 Rincon-Tomasetal2016Josue Santiago LopezNo ratings yet
- Immobilisation of Manganese, Cobalt and Nickel by Deep-Sea-Sediment Microbial CommunitiesDocument18 pagesImmobilisation of Manganese, Cobalt and Nickel by Deep-Sea-Sediment Microbial CommunitiesSujith Puzhambatty PremkumarNo ratings yet
- 9240 Iron and Sulfur Bacteria PDFDocument19 pages9240 Iron and Sulfur Bacteria PDFCalidad LassNo ratings yet
- Synthesis of Copper SilicateDocument4 pagesSynthesis of Copper SilicateBambang WicaksonoNo ratings yet
- Logan y Tagliabue, 2018. Oceanic Micronutrients Trace ElementsDocument6 pagesLogan y Tagliabue, 2018. Oceanic Micronutrients Trace ElementsPaula Patiño TossNo ratings yet
- 9.ISCA IRJBS 2013 199doneDocument9 pages9.ISCA IRJBS 2013 199donevasuki.sNo ratings yet
- Buala, Chapter 11 - Microbial EcologyDocument33 pagesBuala, Chapter 11 - Microbial EcologyJudy Ella BualaNo ratings yet
- 3 - Heavy Metal and Radioactive Waste (Handout)Document27 pages3 - Heavy Metal and Radioactive Waste (Handout)too876555No ratings yet
- Dolly - Mechanism of Sequestration of Heavy Metals by MicroorganismsDocument16 pagesDolly - Mechanism of Sequestration of Heavy Metals by Microorganismsmyacc1603No ratings yet
- 4 HainesDocument7 pages4 HainesFernandoNo ratings yet
- 11 20 Ivelise StrozbergDocument11 pages11 20 Ivelise StrozbergGabriela Wolguemuth MachadoNo ratings yet
- Mechanism of Action and Applications of The Antimicrobial Properties of CopperDocument12 pagesMechanism of Action and Applications of The Antimicrobial Properties of CopperAninditaTrikusumaNo ratings yet
- Bio Leaching ADocument32 pagesBio Leaching AMdhe asif alamNo ratings yet
- MegatDocument8 pagesMegatSITI AISYAH ISHAKNo ratings yet
- Progress in Bioleaching - Fundamentals and Mechanisms of Microbial Metal Sulfide Oxidation - Part ADocument20 pagesProgress in Bioleaching - Fundamentals and Mechanisms of Microbial Metal Sulfide Oxidation - Part AAileen Segura ReimanNo ratings yet
- Bioremediacion Con MicroalgasDocument6 pagesBioremediacion Con MicroalgasbegoNo ratings yet
- Bio Deterioration of Historic Buildings and MonumentsDocument7 pagesBio Deterioration of Historic Buildings and MonumentsDarshi Thamali ParanagamaNo ratings yet
- TD PublishedDocument9 pagesTD PublishedSeptian Perwira YudhaNo ratings yet
- Microbially Induced Calcium Carbonate Precipitation - A WidespreadDocument16 pagesMicrobially Induced Calcium Carbonate Precipitation - A Widespread组换No ratings yet
- Factors Influencing Heavy Metal Removal by Microalgae-A ReviewDocument4 pagesFactors Influencing Heavy Metal Removal by Microalgae-A ReviewsbihiNo ratings yet
- Bacterial Diversity - Nutritional DiversityDocument12 pagesBacterial Diversity - Nutritional DiversityUsman SurajNo ratings yet
- Bio LeachingDocument4 pagesBio LeachingSmitha KollerahithluNo ratings yet
- Data PreprocessingDocument6 pagesData PreprocessingAnonymous VNu3ODGavNo ratings yet
- Bacterial Leaching: - R-ES-O-N - A-N-C-E-I - A-U9-U-s-t - 2-0-04 - 2-7Document8 pagesBacterial Leaching: - R-ES-O-N - A-N-C-E-I - A-U9-U-s-t - 2-0-04 - 2-7Dinesh dhakarNo ratings yet
- Bio Corrosion 2Document20 pagesBio Corrosion 2Kezhakkekarammal Puthiyedattu Sandeep92% (12)
- Festing On Minerals Science 2010Document2 pagesFesting On Minerals Science 2010Simon BeardNo ratings yet
- Adsorption Thermodynamics of Cu (Ii) Ions From Waste Water Using Neem-Leaf Based BiosorbentsDocument8 pagesAdsorption Thermodynamics of Cu (Ii) Ions From Waste Water Using Neem-Leaf Based BiosorbentsArinjayKumarNo ratings yet
- Microbial Lithification in Marine Stromatolites and Hypersaline MatsDocument10 pagesMicrobial Lithification in Marine Stromatolites and Hypersaline MatsCarlos Alquinta PNo ratings yet
- Iron and Manganese CycleDocument10 pagesIron and Manganese Cyclepanda bearNo ratings yet
- Advanced Microbial Ecology: Pre-Msc. StudentsDocument24 pagesAdvanced Microbial Ecology: Pre-Msc. StudentsOmar DoskyNo ratings yet
- Toxic Heavy Metals Dangerous For Life and TheDocument4 pagesToxic Heavy Metals Dangerous For Life and TheInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Biosorption of Heavy Metals: Review PaperDocument8 pagesBiosorption of Heavy Metals: Review Papereagle_snake2002No ratings yet
- Minerals Engineering: Biomining in Reverse Gear: Using Bacteria To Extract Metals From Oxidised OresDocument6 pagesMinerals Engineering: Biomining in Reverse Gear: Using Bacteria To Extract Metals From Oxidised OresMonica DeysiNo ratings yet
- Ca-Carbonates Precipitation and Limestone Genesis - The Microbiogeologist Point of ViewDocument15 pagesCa-Carbonates Precipitation and Limestone Genesis - The Microbiogeologist Point of ViewImrul Kayes KhanNo ratings yet
- Isolation Identification and CharacterizDocument8 pagesIsolation Identification and CharacterizJohnchubyNo ratings yet
- Biodiversity and Ecology of Acidophilic MicroorganismsDocument11 pagesBiodiversity and Ecology of Acidophilic MicroorganismsSrdjan StankovicNo ratings yet
- Jameson 2010Document6 pagesJameson 2010IDELSO JAMIN CHAVEZ CRUZNo ratings yet
- (Brierly, 1978) (Needham and Gwei-Djen, 1974) (Rossi, 1990) (Rossi, 1990)Document5 pages(Brierly, 1978) (Needham and Gwei-Djen, 1974) (Rossi, 1990) (Rossi, 1990)adriel de villaNo ratings yet
- EVOLUTION AssignmentDocument11 pagesEVOLUTION AssignmentDeana NamirembeNo ratings yet
- Photosynthesis and The Carbon Cycle HA&S 220a: C H O + 6O - 6CO 6H ODocument4 pagesPhotosynthesis and The Carbon Cycle HA&S 220a: C H O + 6O - 6CO 6H OSpecsy GuyNo ratings yet
- A Review of BioremediationDocument4 pagesA Review of BioremediationInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Bacterial Ecology PDFDocument7 pagesBacterial Ecology PDFmanoj_rkl_07No ratings yet
- Bacterial Leaching: Biotechnology in The Mining IndustryDocument8 pagesBacterial Leaching: Biotechnology in The Mining IndustryWILLNo ratings yet
- Kiwa Hirsuta "Ye$ Crab?: Size: Distribu, On BiologyDocument46 pagesKiwa Hirsuta "Ye$ Crab?: Size: Distribu, On BiologyAli Mariouni AlawieNo ratings yet
- Heavy Metal - COPPER - CLever, Cobby & GloriaDocument17 pagesHeavy Metal - COPPER - CLever, Cobby & GloriaChisama SichoneNo ratings yet
- Microbial Copper Resistance: Importance in BiohydrometallurgyDocument17 pagesMicrobial Copper Resistance: Importance in BiohydrometallurgyCristóbal Martínez BusseniusNo ratings yet
- Mineral Biotechnology MicrobialDocument272 pagesMineral Biotechnology MicrobialraminNo ratings yet
- Thermodynamic study on adsorption of Copper (II) ions in aqueous solution by Chitosan blended with Cellulose & cross linked by Formaldehyde, Chitosan immobilised on Red Soil, Chitosan reinforced by Banana stem fibreDocument9 pagesThermodynamic study on adsorption of Copper (II) ions in aqueous solution by Chitosan blended with Cellulose & cross linked by Formaldehyde, Chitosan immobilised on Red Soil, Chitosan reinforced by Banana stem fibreijsretNo ratings yet
- Architecture of Biomats Reveals History of Geo-, Aqua-, and Bio-SystemsDocument5 pagesArchitecture of Biomats Reveals History of Geo-, Aqua-, and Bio-Systemsvillamil mNo ratings yet
- BioabsorptionDocument25 pagesBioabsorptionZXaiba Zxafr100% (1)
- Biological Corrosion FailuresDocument10 pagesBiological Corrosion FailuresJose QuiinteroNo ratings yet
- Hydrogeochemical Processes andDocument9 pagesHydrogeochemical Processes andCamila GarcésNo ratings yet
- Laccases: Structure, Reaction, Distribution: Micron February 2004Document5 pagesLaccases: Structure, Reaction, Distribution: Micron February 2004Ştefania RalucaNo ratings yet
- The Winogradsky ColumnDocument4 pagesThe Winogradsky Columnmartincepa100% (1)
- Prepared by Uzochukwu ChidinmaDocument3 pagesPrepared by Uzochukwu ChidinmaMuhammad Tareq AdhaNo ratings yet
- Laura - Bioremediation of Metals - FinalDocument9 pagesLaura - Bioremediation of Metals - Finalapi-343239708No ratings yet
- Newsome Et Al 2014 Microbial U VI ReductionDocument10 pagesNewsome Et Al 2014 Microbial U VI Reductionapi-343239708No ratings yet
- Newsome Et Al 2014 Biogeochemistry ReviewDocument21 pagesNewsome Et Al 2014 Biogeochemistry Reviewapi-343239708No ratings yet
- Newsome Et Al 2015 SerratiaDocument14 pagesNewsome Et Al 2015 Serratiaapi-343239708No ratings yet
- Newsome Et Al 2015 U IV ReoxidationDocument11 pagesNewsome Et Al 2015 U IV Reoxidationapi-343239708No ratings yet
- STFC 1Document1 pageSTFC 1api-343239708No ratings yet
- Newsome NdaDocument1 pageNewsome Ndaapi-343239708No ratings yet
- Newsome Et Al 2015 U IV Phosphate OaDocument9 pagesNewsome Et Al 2015 U IV Phosphate Oaapi-343239708No ratings yet
- Ashima JMLDocument11 pagesAshima JMLPrachi PatnaikNo ratings yet
- Practical Manual: Food Adulteration Prof J.N. SenguptaDocument10 pagesPractical Manual: Food Adulteration Prof J.N. SenguptajnsenguptaNo ratings yet
- Basic Microbiology PracticumDocument11 pagesBasic Microbiology Practicumdiana sihotangNo ratings yet
- Module 1 - Wastewater and Wastewater Treatment Part 1Document37 pagesModule 1 - Wastewater and Wastewater Treatment Part 1Scrappy WellNo ratings yet
- 2 Presentation India Base-Seal Final For 11-04-2016 KLDocument56 pages2 Presentation India Base-Seal Final For 11-04-2016 KLSivaneswaran SabaratnamNo ratings yet
- CAT - Duo Cone Seal InstructionsDocument26 pagesCAT - Duo Cone Seal Instructionsford62b100% (2)
- 303 - Gastrointestinal Physiology) Gastric Secretion - The Cephalic - Gastric PhaseDocument5 pages303 - Gastrointestinal Physiology) Gastric Secretion - The Cephalic - Gastric Phasekedas70No ratings yet
- Specification For Dual-Layer FBEDocument19 pagesSpecification For Dual-Layer FBEali saidNo ratings yet
- Series: Pressure Gauge For General PurposeDocument32 pagesSeries: Pressure Gauge For General Purpose30101985No ratings yet
- Vishay Transducer Application NotesDocument31 pagesVishay Transducer Application Notesxevi00No ratings yet
- Effect of Filler Concentration On Breakdown of IsotacticDocument15 pagesEffect of Filler Concentration On Breakdown of Isotacticdev sNo ratings yet
- STPM Chem Project 4.3 DiscussionDocument3 pagesSTPM Chem Project 4.3 DiscussionXiangjun WooNo ratings yet
- Fisher Vee-Ball v150, v200 and v300 PDFDocument48 pagesFisher Vee-Ball v150, v200 and v300 PDFARMANDONo ratings yet
- D-5381 Kosmetika XRFDocument2 pagesD-5381 Kosmetika XRFTako JankhoteliNo ratings yet
- Standard Specifications For PTFE and Filled PTFEDocument3 pagesStandard Specifications For PTFE and Filled PTFEFaltooNo ratings yet
- Oral MedicationDocument30 pagesOral MedicationPetit NacarioNo ratings yet
- Exercise Chp9Document4 pagesExercise Chp9Siti NorhayatiNo ratings yet
- Oriental Journal of Chemistry 28 (3) 1091-1098Document8 pagesOriental Journal of Chemistry 28 (3) 1091-1098Noureddine BarkaNo ratings yet
- Comparative Study of Natural and Synthetic IndicatorsDocument4 pagesComparative Study of Natural and Synthetic IndicatorsJeff BEzosNo ratings yet
- Gorenje WA 82145 PDFDocument28 pagesGorenje WA 82145 PDFИван АлексиевNo ratings yet
- Characterization of Physicochemical Properties of HydroxypropylDocument8 pagesCharacterization of Physicochemical Properties of HydroxypropylKhoa DuyNo ratings yet
- Recommended Practices For Root Pass Welding of Pipe Without BackingDocument5 pagesRecommended Practices For Root Pass Welding of Pipe Without BackingKENANENo ratings yet
- Practical No 2Document6 pagesPractical No 2shubhamtapryaly9No ratings yet
- The Efficient Way To Prevent Water Carryover and Keep Your Indoor Air HealthierDocument1 pageThe Efficient Way To Prevent Water Carryover and Keep Your Indoor Air HealthierAzrinshah Abu BakarNo ratings yet
- Combinatorial Chemistry: Medicinal Chemistry & Drug Discovery II (MSB)Document34 pagesCombinatorial Chemistry: Medicinal Chemistry & Drug Discovery II (MSB)Md. Abir Hossain100% (3)
- Standard Operating Procedure 12Document3 pagesStandard Operating Procedure 12sindarth raveendrakrishnanNo ratings yet
- Bioenergetics NotesDocument14 pagesBioenergetics NotesMarcela Stevie HadinataNo ratings yet
- Amriks. Pabley, M.D.,And Arthurh. Keeney, M.D.: Louisville, KentuckyDocument8 pagesAmriks. Pabley, M.D.,And Arthurh. Keeney, M.D.: Louisville, KentuckyGun DekNo ratings yet
- Phoenix Metals Product Catalog 2012Document42 pagesPhoenix Metals Product Catalog 2012Norazmi Abdul RahmanNo ratings yet
- Tubing and Casing ConnectionsDocument2 pagesTubing and Casing ConnectionsYougchu LuanNo ratings yet